Experts Detail Potential of 18F-fluciclovine PET/CT to Guide Prostate Cancer Decision-Making
Brian T. Helfand, MD, PhD, and Steven E. Finkelstein, MD, discuss the potential of 18F-fluciclovine PET/CT to guide prostate cancer decision-making.
Brian T. Helfand, MD, PhD
NorthShore Medical Group
Glenview, IL

Steven E. Finkelstein, MD
Florida Cancer Affiliates
Panama City, Florida

Although definitive therapies such as radical prostatectomy and radiation can treat patients with nonmetastatic and localized prostate cancer, 20% to 40% of patients may experience disease progression or inadequate treatment responses.1
Compared with CT and MRI, evidence for 18F-fluciclovine PET/CT demonstrates its ability to provide better diagnostic capabilities for restaging recurrent prostate cancer, with its use in treatment planning typically resulting in a change to salvage radiotherapy following prostatectomy. The synthetic amino acid radiotracer was developed at Emory University and is FDA approved for prostate cancer recurrence imaging.2
In a recent Between the Lines conversation, Brian T. Helfand, MD, PhD, and Steven E. Finkelstein, MD, examined key details from the phase 2/3 randomized EMPIRE-1 trial (NCT01666808) on 18F-fluciclovine PET/CT compared with conventional imaging alone in guiding salvage postprostatectomy radiotherapy for patients with prostate cancer.3
Finkelstein, a radiation oncologist with Florida Cancer Affiliates, part of the US Oncology Network, in Panama City, Florida, said, “We have to phrase this in the mindset [that] most imaging modalities are not approved for improvement in demonstrating cancer outcomes. This is the first time that we are using a PET/radiotracer for prostate cancer, specifically in the setting of postprostatectomy salvage.”
“I think that this is one of the most important articles that people should be aware of, both for our community and academic-based practices,” explained Helfand, a urologist at NorthShore University HealthSystem in Glenview, Illinois. “Most people who are [seeing] this really understand that prostate cancer is a very complex disease and many decisions that we make aren’t perfect.”
The expectation is that, with the integration of 18F-fluciclovine PET/CT into radiotherapy planning, treatment will be more effective compared with standard imaging sequences.
“If you underwent surgery, waiting until your PSA [prostate-specific antigen] value starts to rise from zero can be used to [determine when to] treat disease recurrence. And molecular imaging is one way to help guide us in these treatment decisions,” Helfand explained.
Methods and Results of EMPIRE-1
The study enrolled 165 patients with prostate cancer and randomly assigned them to either conventional imaging only (n = 82) or conventional imaging plus 18Ffluciclovine PET/CT (n = 83).
The primary end point was 3-year event-free survival (EFS), with key secondary end points including pre and postPET treatment decisions and gastrointestinal and genitourinary adverse effects as reported by providers.
“This technology, 18F-fluciclovine PET/CT imaging, has really been used to help us identify or locate targets that can theoretically make subsequent radiation therapy more accurate and improved,” Helfand explained. “We’ve all accepted this as a paradigm: if we can better localize it and we can treat it, then that should give better oncologic outcomes.”
The 3-year EFS analysis found a 12.5% difference between the 2 groups (75.5% vs 63.0%), favoring 18F-fluciclovine-PET/CT vs conventional imaging (95% CI, 4.3%-20.8%; P = .0028).
Variables found to be significant predictors of EFS by univariate analysis included Gleason score greater than 8, absence of extracapsular extension, absence of seminal vesicle invasion, whole pelvic treatment field, and PSA levels of 1.0 ng/mL or more (TABLE).
TABLE. Significant Predictors of EFS by Univariate Analysis
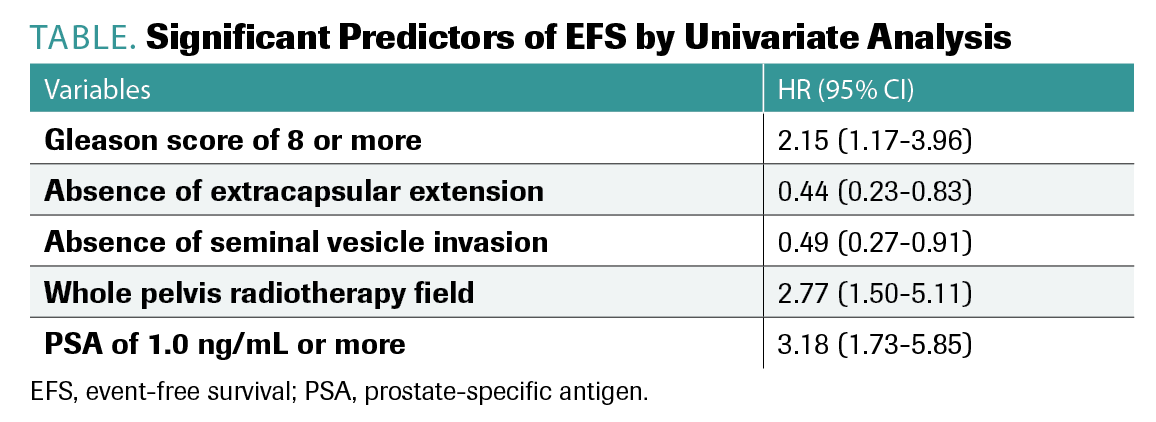
“Once we start analyzing significant predictors and putting this all into a model, we can see that EFS showed significantly higher risk in the conventional imaging group compared with the 18F-fluciclovine group,” Helfand explained.
Along with EFS, a difference of 24.3% (95% CI, 15.6%-33.0%; P < .0001) in failure-free survival at 4 years was observed between the 2 groups, with rates of 75.5% and 51.2% in the 18F-fluciclovine PET/CT and conventional imaging groups, respectively.
Although the trial expanded the typical radiation field delivered to patients, with 25 and 35 patients receiving treatment to the prostate bed and pelvic nodes in the conventional imaging and 18F-fluciclovine PET/CT groups, respectively, that expansion did not correlate with increased grade 3 or higher adverse effects (AEs).
“[In] this group with PET-driven radiation, [we used] larger fields in many [patients], but the toxicity was not significantly higher,” Finkelstein explained. “[I] think urologists will say, ‘I’m excited about the fact that we can deliver PET-targeted radiotherapy and keep the toxicity about the same.’”
AEs were similar between the groups, with common any-grade AEs including late urinary frequency or urgency (46% of patients in the conventional imaging group and 41% of patients the PET group) and acute diarrhea (14% and 21%, respectively). Grade 3 AEs were not frequently observed, and no grade 4 or 5 AEs were reported in either group.
Implications and Key Takeaways
Although many trials have examined the timing of radiotherapy and the roles of androgen deprivation therapy and pelvic lymph node radiation in this setting, this is the first prospective randomized trial to analyze molecular imaging with cancer control as the primary end point.
“[18F-fluciclovine PET/CT] imaging has the ability—and this is the first study to prospectively evaluate this—to change our management and improve overall oncologic outcomes over at least a 3.5-year period without adding additional toxicity,” Helfand said.
Looking ahead, both Helfand and Finkelstein have their fair share of questions regarding radiotherapy modalities used and about newer therapies that are more stereotactically guided. Also, given that this research was a single-institution study conducted at the Winship Cancer Institute of Emory University in Atlanta, Georgia—a center known for developing PET agents—the experts were eager to see how this would translate for the community, according to Helfand.
“As we develop next-generation imaging—whether it is [choline] C-11 PET, Axumin scans, or now [prostate-specific membrane antigen]—the idea of actually finding the targets and then radiating them is much more appealing than just going to radiate where the money potentially is, because we’re only right about half the time,” Finkelstein explained.
Looking ahead, with new next-generation imaging agents on the horizon, Finkelstein hypothesized that the next 10 years will be critical to developing strategies to stratify important variables that may indicate when it is appropriate to accelerate the radiation timeline for patients or to wait to begin the process.
“For the first time, we actually can use a PET-imaging agent, a next-generation agent, and have an improvement using radiation therapy to improve oncologic outcomes,” said Finkelstein.
As the modern management of patients via evidence of disease progression and treatment responses continues to evolve, so will the ability to effectively treat patients with prostate cancer, according to the experts.
“Once we have a true system in place to really stratify patients and understand where that disease is coming from, I think it really changes the field,” Helfand stressed.
References
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433-439. doi:10.1001/jama.294.4.433
- FDA approves new diagnostic imaging agent to detect recurrent prostate cancer. News release. FDA; May 27, 2016. Updated June 8, 2016. Accessed October 8, 2021. https://tinyurl.com/3j6zz665
- Jani AB, Schreibmann E, Goyal S, et al. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial. Lancet. 2021;397(10288):1895-1904. doi:10.1016/S0140-6736(21)00581-X
EP: 1.Phase 2/3 EMPIRE-1 Trial in Prostate Cancer
EP: 2.Prostate Cancer: Clinical Implications From the EMPIRE-1 Trial
EP: 3.Between the Lines Podcast: PET/CT-Guided Postprostatectomy Salvage Radiotherapy for Prostate Cancer
EP: 4.Experts Detail Potential of 18F-fluciclovine PET/CT to Guide Prostate Cancer Decision-Making
Prolaris in Practice: Guiding ADT Benefits, Clinical Application, and Expert Insights From ACRO 2025
April 15th 2025Steven E. Finkelstein, MD, DABR, FACRO discuses how Prolaris distinguishes itself from other genomic biomarker platforms by providing uniquely actionable clinical information that quantifies the absolute benefit of androgen deprivation therapy when added to radiation therapy, offering clinicians a more precise tool for personalizing prostate cancer treatment strategies.