Extreme Case of Surgical Port Metastasis in Ovarian Cancer
We present the case of a 51-year-old woman with metastatic International FIGO stage IIIC ovarian cancer who had delayed her therapy after initial laparoscopy due to COVID-19 infection and presented with an extreme case of surgical port metastasis.
ABSTRACT
Ovarian cancer accounts for more deaths than any other malignancy of the female reproductive system. Early diagnosis of this disease is difficult because there are no systematic opportunistic screening methods. At advanced stages, diagnostic laparoscopy is the first step in confirming disease advancement and obtaining samples for genetic and pathologic examination needed to start chemotherapy. Swiftly starting oncological treatment is crucial for increasing the survival rate in these patients. We present the case of a 51-year-old woman with metastatic International Federation of Gynecology and Obstetrics (FIGO) stage IIIC ovarian cancer who had delayed her therapy after initial laparoscopy due to COVID-19 infection and presented with an extreme case of surgical port metastasis.
Ovarian cancer is one of the most common malignant diseases in women. The highest rates of new cases (11.4 per 100,0000) and deaths (6.0 per 100,0000) are seen in Eastern and Central Europe.1 Looking at cancer-related deaths in the world, ovarian cancer ranks fifth, and for
cancer-related deaths in Poland, it ranks fourth.2,3 Worldwide, the disease typically presents in an advanced stage, when the 5-year survival rate is 29%.1 Two-thirds of patients present with stage III or stage IV disease, which means upfront surgery is often not feasible.4 Many patients require diagnostic laparoscopy to determine tumor genetic and histopathological status and neoadjuvant chemotherapy before cytoreductive surgery. The prolonged interval between laparoscopic biopsy and chemotherapy may lead to additional comorbidities.5
Case Presentation
In January 2023 a 51-year-old woman, gravida 3, para 2, was admitted to ambulatory care with symptoms of ascites. Her medical history included mild arterial hypertension, currently without treatment. One year ago, the patient was referred for ovarian cyst surgery (uniocular, anechoic, 7-cm diameter) but did not report for the procedure. The patient reported 10-kg weight loss in the previous 6 months. The risk of ovarian malignancy algorithm was 79%. During the physical examination, palpable solid lesions in the pelvis were found. On the transvaginal ultrasound scan, a solid cystic lesion measuring 11.0 cm×9.0 cm×7.5 cm was detected in the pelvis. The transabdominal ultrasound scan confirmed free fluid in the peritoneum and detected omental cake.
CT scans of the pelvis, abdomen, and thorax were performed. The CT scan found extensive pathological nodular lesions with a fluidlike structure extending from the level of the umbilicus to the pelvis in communication with the right adnexa. The lesion measured 19.0 cm×11.5 cm×14.5 cm. The thickness of the lesion at the anterior abdominal wall was approximately 3.5 cm. Numerous tumor implants in the greater omentum, along the wall of the small intestine, were suspected. Tumor implants and enlarged lymph nodes were suspected in the mesentery; there was a hypodense lesion in the sixth segment of the liver that measured 3.5 cm×3.5 cm and an osteolytic lesion in the ischium with a diameter of 2.0 cm. The patient was qualified for laparoscopy with the intent of biopsy of a suspicious lesion.
During the surgery, node lesions in the corpus uteri, thick green fluid in the peritoneal cavity, and nodular implants in the liver recess, splenic recess, omentum, and peritoneum were found. The Fagotti score was 14 points.6 A total of 120 mL of fluid from the peritoneal cavity, tissue slices from the omentum, and implants from the peritoneum were taken for histopathological and genetic examination.
The bleeding sites were coagulated, and a surgical drain was placed in the rectovaginal pouch. Surgical sutures were placed in the rectus sheath at the trocar site near the umbilicus and on the other 2 trocar sites. Single interrupted surgical sutures were placed in the skin.
During a histopathology examination, metastases of epithelial cancer and carcinosarcoma were found. Immunohistochemistry was positive for CK7, p53, PAX9, VIM, and CK19. Morphologically epithelial and spindle cells also were found.
The patient was urgently referred for chemotherapy, and her appointment in the clinical oncology department was scheduled 2 weeks after laparoscopy. Due to a viral infection of the upper respiratory tract and influenza, the patient was temporarily disqualified and did not return for reevaluation 2 weeks later.
In March 2023, the patient was admitted to the gynecologic emergency department. She had not returned to clinical oncology due to further COVID-19 infection and personal issues. In the physical examination, a new lesion measuring approximately 5.0 cm×6.0 cm was found in the umbilical region. After removing the dressing from the umbilicus, attention was drawn to the bleeding from the lesion (Figure 1).
FIGURE 1. Tumor Implants in the Umbilicus.
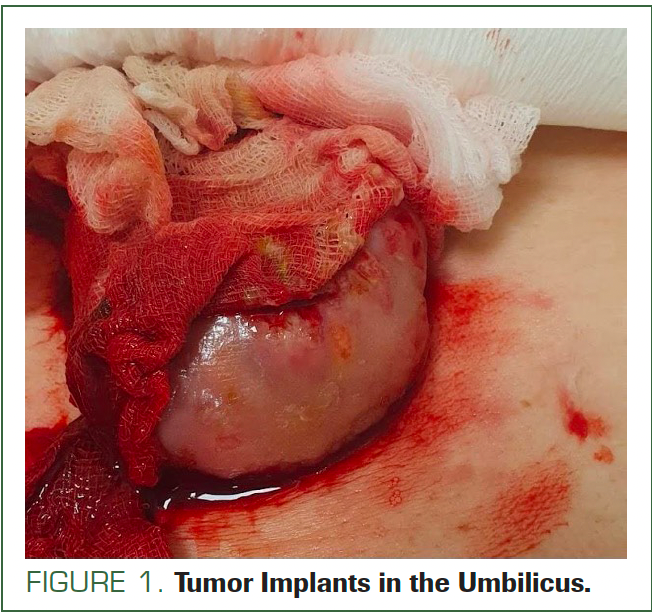
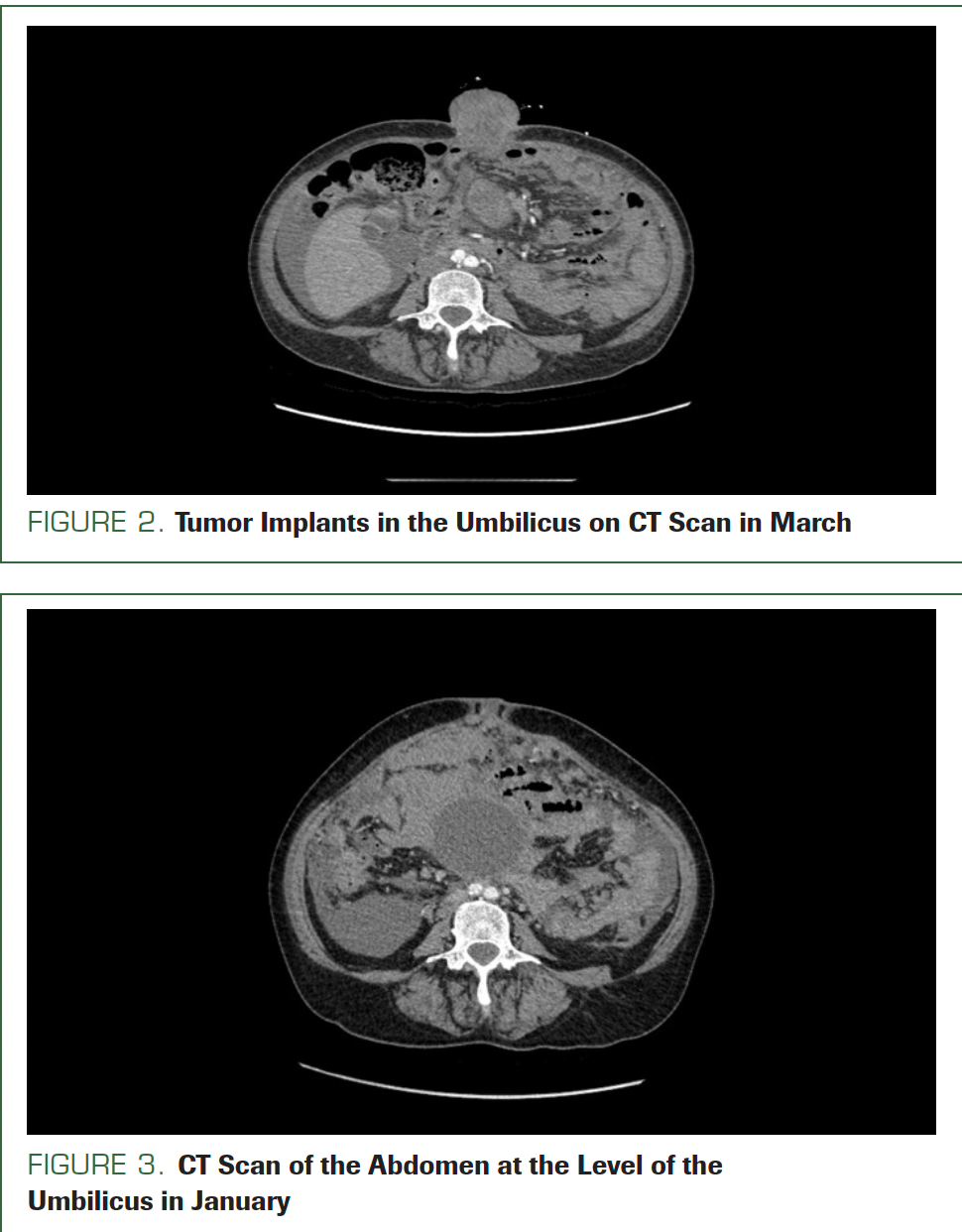
Umbilical jejunum hernia or metastasis of primary disease was taken into consideration. From the emergency department, the patient was referred for CT with contrast. The CT scans, compared with scans from the first admission, showed the thickness of the lesion at the anterior abdominal wall was 4.0 cm; it was 3.5 cm in January. Additionally, the tumor implant in the umbilicus now measured 5.2 cm × 6.3 cm (Figure 2); in January it was 1.9 cm × 1.8 cm (Figure 3).
FIGURE 2. Tumor Implants in the Umbilicus on CT Scan in March
FIGURE 3. CT Scan of the Abdomen at the Level of the Umbilicus in January
The patient’s C-reactive protein level was 205 mg/L, hemoglobin level was 7.9 g/dL, and leukocyte level was 16.5 μL. Due to anemia, 2 units of packed red blood cells were transfused. After surgical consultation, the authors decided to abandon the surgical treatment. The patient was referred for immediate radiation treatment of the tumor in the umbilicus and systemic chemotherapy.
Discussion and Review of the Literature
Ovarian cancer often presents with liver and splenic parenchymal metastases that contribute to International Federation of Gynecology and Obstetrics (FIGO) stage IV disease. This advanced type of cancer is present in 12% to 33% of the patients at initial diagnosis.7 Study reports show the sites of distant metastases of ovarian cancer are pleural effusion/pulmonary metastases (ca. 40% of patients); abdominal wall metastases (ca. 40% of patients with FIGO stage IV disease); extra-abdominal lymph nodes (ca. 20%); liver metastases (ca. 14%); spleen metastases (ca. 6%); brain metastases (ca. 2%), and bone involvement (<2%).8-10 Findings on the patient’s CT scans, including tumor implants in the greater omentum and lesion in the liver, raised the suspicion of distant metastases of the disease; nevertheless, a laparoscopic biopsy would be needed to confirm the disease by histological examination of the lesion. Also, assessment of the extension of the disease during laparoscopy influences the primary treatment option for the patient.5,11
One of the most frequently used tools in the prediction of optimal cytoreduction is the Fagotti score. In this model, we consider the following parameters: omental cake, peritoneal carcinomatosis, diaphragmatic carcinomatosis, mesenteric involvement, bowel infiltration, stomach infiltration, and liver metastases.6 The predictive index value for each positive parameter is 2. If the cut value is 8 or more, then the probability of converting the laparoscopy to laparotomy and optimal cytoreduction (residual tumor ≤ 1.0 cm) is equal to 0.12 The presence of omental cake, peritoneal and diaphragmatic extensive carcinosis, mesenteric retraction, bowel and stomach infiltration, spleen and/or liver superficial metastasis was investigated by laparoscopy. By summing the scores relative to all parameters, a laparoscopic assessment for each patient was evaluated (total predictive index value = PIV).
Recently, more often the real destination of surgical treatment is complete cytoreduction (residual tumor = 0 cm). Then the cut value of Fagotti score is 10 or more points.13
In the literature, we found potential risk factors for abdominal wall metastases after laparoscopic surgery: FIGO stage IV disease, ascites volume higher than 500 mL, and peritoneal carcinomatosis.14 These factors are closely correlated with the mechanism of the port-site recurrences. The starting point is when tumor cells are present within the abdominal cavity. The peritoneal wound by laparoscopic instruments breaches the mechanical protection provided by the mesodermal layer. Finally, the implantation of tumor cells into the laparoscopic port site could occur by direct contact, direct inoculation via laparoscopic instruments, or through contaminated peritoneal liquid after the procedure. Additionally, local conditions in the abdominal wound, such as angiogenic growth factors, cell growth factors, and the presence of inflammation mediators, promote the growth of the tumor cells.15
The stimulatory effect of carbon dioxide (CO2) on abdominal wall metastasis has a secondary role in clinical practice. Although we found a few studies in animal models showing that using gasless laparoscopy reduced the incidence of port-site recurrences, after thoracoscopy (during which no CO2 is used) numerous port-site recurrences have also been described.16,17 The presence of the tumor cells in the port sites is instead a result of direct contamination by the surgical instruments and not from the dispersal of the malignant cells by the CO2.18,19The benefits of using gasless laparoscopy procedures remain controversial, and further research is needed in this area.
The other proposed way of dissemination of malignant cells to the abdominal wound is hematogenous spread. It might be that higher intra-abdominal pressure during laparoscopy facilitates the passage of malignant cells from the lymphatic to the venous system and causes implantation of neoplasm cells at trocar sites.20 However, the other authors proved that only 1% of neoplasm cells that reach general circulation survive, and only 0.1% of these cells can cause metastases. Additionally, this mechanism does not explain the difference in wound metastases after laparoscopy and laparotomy, and the number of reported cases of metastases after open surgery is not as high as this mechanism would suggest.21
The incidence of abdominal wall metastases after diagnostic procedures varies among available scientific reports. We found 1 report that noted abdominal wall metastases appear in 47% of patients after laparoscopic interventions.14 Another study proved that the risk of port-site metastases is 50% in patients with ovarian and primary peritoneal malignancies in the presence of ascites.22 Other studies’ results showed that the risk of port-site metastases is not as high as in the studies mentioned above: port-site metastases in 1 of 88 patients (1.14% per procedure) undergoing laparoscopic surgery for ovarian cancer and 1% per procedure for gynecologic malignancies in general.23 However, Kruitwagen et al reported that the incidence of port-site metastases is 16% in patients with ovarian cancer undergoing laparoscopic procedures 9 to 35 days prior to the initial cytoreduction.24
When analyzing the articles and describing the correlation between abdominal wall metastases and risk factors, we found factors that could reduce the risk of recurrence of the disease at the port sites. In the study conducted by Van Dam et al, patients with abdominal implantations had a longer interval between primary laparoscopy and the debulking surgery or the start of chemotherapy.25 Abdominal implantations developed in 0 patients in which cytoreductive surgery or chemotherapy was done within 1 week after a diagnostic laparoscopic procedure. The early onset of postoperative chemotherapy is correlated with the lower risk of the occurrence of port-site metastases.26,27
Van Dam et al also proved that proper surgical technique is very important.25 Trocar site metastases appear in 58% of patients who underwent a closed laparoscopic procedure with a blunt trocar; the peritoneum and rectus sheath were not closed at the end of the operation, and the sutures were placed on the skin. Trocar site implantation metastases were found in only 2% of patients in whom an open laparoscopy was performed with careful closure of all layers of the abdominal wall.28 An additional proven risk factor for early occurrence of port-site metastasis is the presence of ascitic fluid during the laparoscopic procedure.28 Aspiration of all intraabdominal fluid before trocar removal, abdominal emphysema removal with trocar in place, and proper trocar fixation are other general recommendations proved in the scientific literature.29
In the other animal model, a significant reduction in port-site metastases was observed, when diluted povidone-iodine was instilled in the peritoneal cavity during laparoscopic procedure.30
A decrease in port-site metastases was also observed in the rat model of colon cancer. Irrigating the port sites with 5-fluorouracil at the time of procedure led to a decrease in occurrence of port-site metastases.31 The harmfulness of irritative substances for the tissue and the benefits of minimizing the risk of port-site recurrences should be considered before using these agents.32
Port-site resection was another potential option to minimize the occurrence of port-site metastases. The authors analyzed the correlation between port-site resection and oncological outcome in advanced ovarian cancer: No better outcome in survival and higher prevalence of wound complications were observed.33
From the factors mentioned above, the delay of postoperative chemotherapy and the presence of ascites were the risk factors for developing port-site metastasis. The use of proper surgical technique of laparoscopy and proper wound closure in layers (rectus, sheet, and skin) did not protect the abdominal wall from metastasis in the place of the trocar site.
Conclusions
Although neoadjuvant chemotherapy may benefit patients in advanced stages of ovarian cancer, it is important to plan the treatment at the proper place after laparoscopic biopsy. Additionally, physcians and patients should make every effort not to delay proper therapy after the first stage of treatment, which is a diagnostic laparoscopy.
Author affiliations
1Department of Gynecology and Gynecological Oncology, Center for Postgraduate Medical Education, 01-809, Warsaw, Poland
Corresponding author
Michał Kostrzanowski, MD
Department of Gynecology and Gynecological Oncology, Center for Postgraduate Medical Education, 01-809, Warsaw, Poland
Phone: +48 790-231-177
Email:mkostrzan@gmail.com
Acknowledgment and disclosures
We wanted to express our gratitude to everyone who helped during the writing of this case report.
References
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14(1):9-32. doi:10.20892/j.issn.2095-3941.2016.0084
- American Cancer Society. Key statistics for ovarian cancer. Accessed January 24, 2023. https://shorturl.at/aguLS
- Didkowska J, Wojciechowska U, Michalek IM, Caetano Dos Santos FL. Cancer incidence and mortality in Poland in 2019. Sci Rep. 2022;12(1):10875. doi:10.1038/s41598-022-14779-6
- Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet. 2021;155(suppl 1):61-85. doi:10.1002/ijgo.13878
- Coleridge SL, Bryant A, Kehoe S, Morrison J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2021;7(7):CD005343. doi:10.1002/14651858.CD005343.pub6
- Fagotti A, Ferrandina G, Fanfani F, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol. 2006;13(8):1156-1161. doi:10.1245/ASO.2006.08.021
- Ataseven B, Chiva LM, Harter P, Gonzalez-Martin A, du Bois A. FIGO stage IV epithelial ovarian, fallopian tube and peritoneal cancer revisited. Gynecol Oncol. 2016;142(3):597-607. doi:10.1016/j.ygyno.2016.06.013
- Ataseven B, Grimm C, Harter P, et al. Prognostic impact of debulking surgery and residual tumor in patients with epithelial ovarian cancer FIGO stage IV. Gynecol Oncol. 2016;140(2):215-220. doi:10.1016/j.ygyno.2015.12.007
- Ogawa K, Yoshii Y, Aoki Y, et al. Treatment and prognosis of brain metastases from gynecological cancers. Neurol Med Chir (Tokyo). 2008;48(2):57-63. doi:10.2176/nmc.48.57
- Cormio G, Rossi C, Cazzolla A, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003;13(2):125-129. doi:10.1046/j.1525-1438.2003.13054.x
- Brun JL, Rouzier R, Selle F, Houry S, Uzan S, Daraï E. Neoadjuvant chemotherapy or primary surgery for stage III/IV ovarian cancer: contribution of diagnostic laparoscopy. BMC Cancer. 2009;9:171. doi:10.1186/1471-2407-9-171
- Fagotti A, Ferrandina G, Fanfani F, et al. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am J Obstet Gynecol. 2008;199(6):642.e1-642.e6426. doi:10.1016/j.ajog.2008.06.052
- Petrillo M, Vizzielli G, Fanfani F, et al. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: proof of a concept. Gynecol Oncol. 2015;139(1):5-9. doi:10.1016/j.ygyno.2015.07.095
- Heitz F, Ognjenovic D, Harter P, et al. Abdominal wall metastases in patients with ovarian cancer after laparoscopic surgery: incidence, risk factors, and complications. Int J Gynecol Cancer. 2010;20(1):41-46. doi:10.1111/IGC.0b013e3181c443ba
- Reymond MA, Schneider C, Kastl S, Hohenberger W, Köckerling F. The pathogenesis of port-site recurrences. J Gastrointest Surg. 1998;2(5):406-414. doi:10.1016/s1091-255x(98)80030-9
- Bouvy ND, Marquet RL, Jeekel H, Bonjer HJ. Impact of gas(less) laparoscopy and laparotomy on peritoneal tumor growth and abdominal wall metastases. Ann Surg. 1996;224(6):694-701. doi:10.1097/00000658-199612000-00005
- Fry WA, Siddiqui A, Pensler JM, Mostafavi H. Thoracoscopic implantation of cancer with a fatal outcome. Ann Thorac Surg. 1995;59(1):42-45. doi:10.1016/0003-4975(94)00794-8
- Thomas WM, Eaton MC, Hewett PJ. A proposed model for the movement of cells within the abdominal cavity during CO2 insufflation and laparoscopy. Aust N Z J Surg. 1996;66(2):105-106.
- Reymond MA, Wittekind C, Jung A, Hohenberger W, Kirchner T, Köckerling F. The incidence of port-site metastases might be reduced. Surg Endosc. 1997;11(9):902-906. doi:10.1007/s004649900483
- Targarona EM, Martínez J, Nadal A, et al. Cancer dissemination during laparoscopic surgery: tubes, gas, and cells. World J Surg. 1998;22(1):55-61. doi:10.1007/s002689900349
- Gutman M, Fidler IJ. Biology of human colon cancer metastasis. World J Surg. 1995;19(2):226-234. doi:10.1007/BF00308631
- Nagarsheth NP, Rahaman J, Cohen CJ, Gretz H, Nezhat F. The incidence of port-site metastases in gynecologic cancers. JSLS. 2004;8(2):133-139.
- Childers JM, Aqua KA, Surwit EA, Hallum AV, Hatch KD. Abdominal-wall tumor implantation after laparoscopy for malignant conditions. Obstet Gynecol. 1994;84(5):765-769.
- Kruitwagen RF, Swinkels BM, Keyser KG, Doesburg WH, Schijf CP. Incidence and effect on survival of abdominal wall metastases at trocar or puncture sites following laparoscopy or paracentesis in women with ovarian cancer. Gynecol Oncol. 1996;60(2):233-237. doi:10.1006/gyno.1996.0031
- van Dam PA, DeCloedt J, Tjalma WA, Buytaert P, Becquart D, Vergote IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. 1999;181(3):536-541. doi:10.1016/s0002-9378(99)70489-8
- Leminen A, Lehtovirta P. Spread of ovarian cancer after laparoscopic surgery: report of eight cases. Gynecol Oncol. 1999;75(3):387-390. doi:10.1006/gyno.1999.5596
- Lécuru F, Daraï E, Robin F, Housset M, Durdux C, Taurelle R. Port site metastasis after laparoscopy for gynecological cancer: report of two cases. Acta Obstet Gynecol Scand. 2000;79(11):1021-1023.
- Wang PH, Yuan CC, Lin G, Ng HT, Chao HT. Risk factors contributing to early occurrence of port site metastases of laparoscopic surgery for malignancy. Gynecol Oncol. 1999;72(1):38-44. doi:10.1006/gyno.1998.5128
- Ramirez PT, Wolf JK, Levenback C. Laparoscopic port-site metastases: etiology and prevention. Gynecol Oncol. 2003;91(1):179-189. doi:10.1016/s0090-8258(03)00507-9
- Neuhaus SJ, Watson DI, Ellis T, Dodd T, Rofe AM, Jamieson GG. Efficacy of cytotoxic agents for the prevention of laparoscopic port-site metastases. Arch Surg. 1998;133(7):762-766. doi:10.1001/archsurg.133.7.762
- Eshraghi N, Swanstrom LL, Bax T, et al. Topical treatments of laparoscopic port sites can decrease the incidence of incision metastasis. Surg Endosc. 1999;13(11):1121-1124. doi:10.1007/s004649901186
- Lee BR, Tan BJ, Smith AD. Laparoscopic port site metastases: incidence, risk factors, and potential preventive measures. Urology. 2005;65(4):639-644. doi:10.1016/j.urology.2004.09.067
- Lago V, Gimenez L, Matute L, et al. Port site resection after laparoscopy in advance ovarian cancer surgery: time to abandon? Surg Oncol. 2019;29:1-6. doi:10.1016/j.suronc.2019.01.007

Elevating the Quality of Cancer Care via Cross-Department Collaboration
Experts from Sibley Memorial Hospital discuss how multidisciplinary work has enhanced outcomes such as survival and resource use at their institution.