FDA Approves Tarceva in Second-Line Tx
This special “annual highlights” supplement to Oncology News International is acompilation of major advances in the management of lung cancer during 2004, asreported in ONI. Guest editor Dr. Roy Herbst discusses these advances in clinicalmanagement, with a focus on developments in adjuvant therapy for early disease,targeted therapy, and new chemotherapy findings.
ROCKVILLE, MarylandThe Food and Drug Administration(FDA) has approved Tarceva tablets(erlotinib, OSI Pharmaceuticals) as asingle-agent treatment for patientswith locally advanced or metastaticnon-small-cell lung cancer (NSCLC)whose disease has continued toprogress despite other therapies, includingat least one prior chemotherapyregimen. The FDA granted marketingapproval on the basis ofincreased survival findings in theTarceva-treated arm of a phase III trialin patients who had received second-or third-line therapy for advanced NSCLC. The primary endpointof the study was overall survival.Secondary endpoints includedprogression-free survival and tumorresponse rate.Tarceva, developed by OSI anddistributed by Genentech, is an epidermalgrowth factor receptor (EGFR)inhibitor, and it is the first of the classto demonstrate a survival advantagefor advanced NSCLC in a phase IIItrial. However, two large, randomizedstudies of Tarceva given concurrentlywith doublet platinum-basedchemotherapy in previously untreatedadvanced NSCLC failed to show clinical benefit, and the drug is notrecommended for such use.The pivotal study involved 731 patientswith locally advanced or metastaticNSCLC in a randomized,double-blind, placebo-controlledtrial conducted in 17 countries. Patientswere randomized 2:1 to receiveTarceva 150 mg or placebo, administeredorally once daily until diseaseprogression or unacceptable toxicity.Survival, progression-free survival,and tumor response all proved significantin favor of Tarceva (P < .001).Median survival, evaluated in an intent-to-treat population, was 6.7months for Tarceva vs 4.7 months forplacebo. Median progression-free survivalwas 9.9 vs 7.9 weeks, respectively,and the Tarceva-treated patients had a tumor response rate of 8.9% vs0.9% for placebo. One-year survivalwas 31.2% in the treatment group vs21.5% for the control patients, andmedian duration of response was 34.3vs 15.9 weeks, respectively.
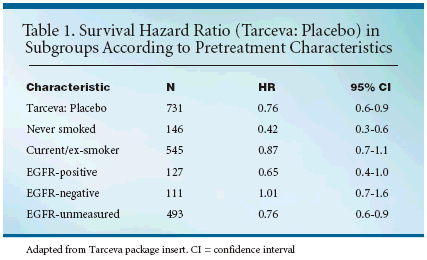
EGFR status was not part of thestudy protocol, and survival was seenin a broad range of patients. Survivalamong EGFR-positive patients waslooked at retrospectively, however.Among the 238 patients with a knownEGFR status, the 78 EGFR-positiveTarceva-treated patients had a bettersurvival rate than the 49 EGFR-positiveplacebo patients. Tarceva did notappear to affect survival in the EGFRnegativesubgroup (HR = 1.01). Asurvival benefit due to Tarceva in theEGFR-negative subgroup cannot beexcluded, however, because the confidenceintervals for the EGFR-positive,-negative, and unmeasuredsubgroups are wide and overlap (seeTable 1).The most common adverse reactionsin patients receiving Tarcevawere rash and diarrhea.