First Medullary Thyroid Cancer Drug Is Approved
The US Food and Drug Administration approved the first treatment for a rare type of thyroid cancer on Friday April 6, 2011.
The US Food and Drug Administration (FDA) approved the first treatment for a rare type of thyroid cancer on Friday April 6, 2011. Vandetanib, which has no official brand name as of yet, is made by AstraZeneca. Vandetanib is an orphan drug for patients with late-stage medullary cancer of the thyroid who are not eligible for surgery.
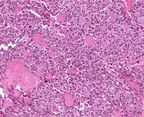
Slide from a 28-year-old male with medullary thyroid cancer. Source:
Robert Cardiff, MD, PhD
The incidence of medullary thyroid cancer in 2010 was approximately 1,300 to 2,200 patients in the United States and represents 3% to 5% of all thyroid cancers, according to National Cancer Institute statistics. A total of 44,600 new thyroid cancer cases were diagnosed in the United States last year, and about 1,690 patients died from the disease. Thyroid cancer affects the thyroid gland in the neck; medullary thyroid cancer affects specific cells in the gland and can occur spontaneously or be part of a genetic syndrome.
Vandetanib is an oral kinase inhibitor that is taken daily. “Vandetanib is the only medicine to receive FDA approval specifically for use in patients with advanced medullary thyroid cancer and is the first treatment that AstraZeneca has developed and brought to market under orphan drug designation in the United States,” said Howard Hutchinson, MD, chief medical officer at AstraZeneca.
Trial Results That Led to Approval
The FDA approved the kinase inhibitor based on the results of a 331-patient, randomized phase III trial called the ZETA study. The study showed a 65% risk reduction in disease progression in the drug arm compared to placebo. The median progression-free survival on vandetanib was 22.6 months, compared to 16.4 months in the control arm. To date, it is still unknown whether vandetanib affects overall survival of patients with medullary thyroid cancer.
Patients with asymptomatic or slow-progressing disease should carefully consider the risk-benefit ratio, as the treatment has substantial side effects. The boxed warning for vandetanib includes QT prolongation, torsades de pointes, and sudden death. The FDA has therefore required a risk-evaluation and mitigation strategy (REMS) that focuses on serious heart-related risks. Only clinicians and pharmacies that are part of the REMS program are able to administer the medication. Common side effects exhibited in more than 20% of patients were diarrhea, rash, acne, nausea, hypertension, headache, fatigue, decreased appetite, and abdominal pain.
In December 2010, an FDA advisory board recommended approval of vandetanib, but the drug approval date was moved forward 3 months as the REMS plan was being carved out. The drug was originally studied for advanced non–small-cell lung cancer, but a late-stage trial failed to show an overall survival when a 100-mg dose was combined with chemotherapy.
“Vandetanib’s approval underscores FDA’s commitment to approving treatments for patients with rare and difficult to treat diseases,” said Richard Pazdur, MD, director of the Office of Oncology Drug Products in the FDA’s Center for Drug Evaluation and Research, in a press release. The orphan drug is still under regulatory review in Europe and Canada.