FOLFOXIRI Bests FOLFIRI for Metastatic Colorectal Ca
Compared with FOLFIRI, the FOLFOXIRI regimen increases the rate of radical resection, prolongs time to progression, and may improve survival when used as first-line therapy for metastatic colorectal cancer, new data show. Alfredo Falcone, MD, professor of medical oncology, University of Pisa, and chairman of Oncology, Livorno Hospital, presented the findings at the 2006 Gastrointestinal Cancers Symposium (abstract 227).
SAN FRANCISCOCompared with FOLFIRI, the FOLFOXIRI regimen increases the rate of radical resection, prolongs time to progression, and may improve survival when used as first-line therapy for metastatic colorectal cancer, new data show. Alfredo Falcone, MD, professor of medical oncology, University of Pisa, and chairman of Oncology, Livorno Hospital, presented the findings at the 2006 Gastrointestinal Cancers Symposium (abstract 227). Patients were eligible for the phase III trial, conducted by the Italian Northwest Oncology Group (GONO), if they had nonresectable metastatic colorectal cancer and had not received any chemotherapy for advanced disease. They were randomly assigned to treatment with the "doublet" regimen of FOLFIRIirinotecan (Camptosar) plus infusional fluorouracil (5-FU)/leucovorinor with the "triplet" regimen of FOLFOXIRIthe same combination with added oxaliplatin (Eloxatin). The planned treatment was 12 cycles given at 2-week intervals. If patients in the FOLFIRI arm had a progression, oxaliplatin-containing chemotherapy was recommended as second-line therapy.
A total of 244 patients were enrolled. Roughly one-quarter had received adjuvant chemotherapy. Half had multiple sites of metastases, while about one-third had metastases only in the liver, Dr. Falcone said. Relative dose intensity was between 80% and 90% in both treatment arms, although colony-stimulating factors were more frequently used in the FOLFOXIRI group, he said. Median follow-up was 15.2 months.
In intention-to-treat analyses, the overall rate of response (complete or partial) was significantly higher in the patients on FOLFOXIRI than in those on FOLFIRI (66% vs 41%, P = .0002).
"This increased activity of the treatment allowed a greater number of patients to undergo a radical resection of residual metastases," Dr. Falcone noted. Specifically, he said, the proportion of patients who were able to have an R0 resection was significantly greater in the FOLFOXIRI group (15% vs 6%, P = .033). "So disease was eradicated with a combination of chemotherapy and surgery. We know this is potentially important because some of these patients have long-term survival and some can even be cured," he added. Moreover, he said, when analyses were restricted to the patients who had only liver metastases, the proportion able to undergo R0 resection was a significant threefold higher in the FOLFOXIRI arm (36% vs 12%, P = .017).
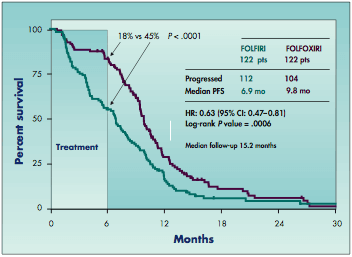
Progression-free survival (PFS) in Italian GONO trial of FOLFIRI (irinotecan/5-FU/leucovorin) vs FOLFOXIRI (irinotecan/5-FU/leucovorin/oxaliplatin).
After 6 months (the time at which chemotherapy was stopped), 45% of patients in the FOLFIRI group had experienced progression, compared with 18% of their counterparts in the FOLFOXIRI group (see Figure). Median progression-free survival was 6.9 months with the double regimen vs 9.8 months with the triple one. The difference corresponded to a significant one-third reduction in the risk of progression (hazard ratio 0.63, P = .0006).
Although survival data are still immature, Dr. Falcone cautioned, 21% of patients in the FOLFIRI group are still alive after 30 months, compared with 34% of those in the FOLFOXIRI group. Median overall survival is 16.7 and 22.6 months, respectively, a difference corresponding to a significant 30% reduction in the risk of death (hazard ratio 0.70, P = .032).
"Overall, there was a modest increase in toxicity with FOLFOXIRI," Dr. Falcone said. About 50% of FOLFOXIRI patients developed grade 3-4 neutropenia vs about 28% of those in the FOLFIRI arm. However, the respective incidences of febrile neutropenia were 5% and 3%, and were statistically indistinguishable. And, as expected based on the known toxicity profile of oxaliplatin, he said, patients in the FOLFOXIRI arm also had a higher incidence of grade 2-3 peripheral neurotoxicity (20%) than those in the FOLFIRI arm (0%). The rate of grade
3-4 diarrhea tended to be higher with the triple regimen (20% vs 12%). The overall incidence of serious adverse events was similar18% with FOLFOXIRI and 20% with FOLFIRIand none of the patients died from toxicities.
Adding Bevacizumab
"The FOLFOXIRI regimen we studied was feasible with manageable toxicity, also in a multicenter setting," and achieved improvements in a variety of clinical outcomes, Dr. Falcone concluded. "Today, the standard is not anymore just a [chemotherapy] doublet . . . but a doublet plus bevacizumab [Avastin], either with FOLFIRI or FOLFOX," he noted. "Results obtained with FOLFOXIRI look similar to those reported with a doublet plus bevacizumab." Therefore, he stated, a trial testing bevacizumab added to FOLFOXIRI "should be feasible, and I think it should be done."