Granulocytic Sarcoma in a Patient With Myelodysplastic Syndrome
Our case illustrates the fact that MDS-associated GS can be treated palliatively with radiation and hypomethylating agents in an appropriate setting. With the growing geriatric patient population, effective treatment options are needed in this disease.
ABSTRACT: Granulocytic sarcomas (GS) are uncommon extramedullary tumors composed of immature cells of the granulocytic or myeloid series. Treatment for GS should be directed toward the underlying hematologic disorder. There is no standard treatment.
Granulocytic sarcoma (GS) is seen in ~5% of myeloid leukemia cases and represents the "tissue phase" of the disease. GS can also be seen in patients with chronic myeloproliferative disorders or myelodysplastic syndromes (MDS).[1,2] It antedates the development of acute myeloid leukemia (AML) by about 1 to 2 years.[2] Common sites of involvement include the skin, gums, lymph nodes, soft tissues, periosteum, and bone. Involvement of the gastrointestinal (GI) tract is very rare and commonly involves the small intestine presenting with intestinal obstruction or perforation.[3-5] Gastric GS may occur as gastric ulcers or polyps, and patients most often present with an acute GI bleed.[4-6]
Well-differentiated GS consists of tumor cells of myeloid origin showing various stages of maturation. The specific esterase stain, chloroacetate esterase (also called Leder stain), stains neutrophils, neutrophil precursors, eosinophils, and mast cells and confirms the granulocytic nature of the tumor. Various karyotypic abnormalities are seen in primary GS or AML with GS such as t(8,21), 11q23, and inv(16).[7-9] Molecular analysis such as fluorescence in situ hybridization (FISH) can also help detect specific cytogenetic abnormalities.
Treatment for GS should be directed toward the underlying hematologic disorder, but there is no standard treatment. Treatment of symptomatic GI tract GS poses a special challenge. Most physicians treat granulocytic sarcoma as AML using standard chemotherapy regimens.[10] However, care should be taken to avoid premature induction therapy, since treatment of MDS-associated GS does not prolong survival. Patients with MDS, who develop GS as a marker of disease acceleration and transformation to AML, have a poor prognosis and survival despite aggressive chemotherapy.[11,12]
Case Report
FIGURE 1
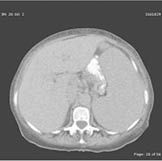
CT Scan of the Abdomen-Computed tomography (CT) scan of the abdomen showing a mass in the gastric antrum, massive splenomegaly, and extensive lymphadenopathy.
A 67-year-old woman was diagnosed with refractory anemia with excess blasts II (RAEB II, 13%) in 2005. She was treated on protocol with thalidomide (Thalomid) and azacitadine (Vidaza). Upon disease progression, she enrolled in another clinical trial and was treated with a monoclonal antibody to CD33. While on treatment she presented with a 1-week history of melena, nausea, decreased appetite, abdominal pain, weight loss, and fatigue. She was hypotensive and tachycardic, with abdominal tenderness and splenomegaly. Her hematocrit was 20%, hemoglobin 6 g/dL, platelet count 59,000/mm3, and white blood cell count 71,000 /mm3, with 9.3% blasts on the peripheral smear.
She was admitted to the intensive care unit in February 2007 and was initially thought to have GI bleeding secondary to thrombocytopenia, and platelet dysfunction secondary to MDS. She received a platelet transfusion. Because of the abdominal pain, splenomegaly, and low hematocrit on the initial presentation, a computed tomography scan of the abdomen was performed, showing a 6.4 - 5.5 cm mass in the gastric antrum, massive splenomegaly of 21.5 - 8.5 cm, and extensive lymphadenopathy (Figure 1).
FIGURE 2
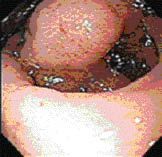
Endoscopic Image-Endoscopy revealing a large submucosal, noncircumferential mass in the gastric antrum.
Endoscopy showed a large submucosal, noncircumferential mass in the gastric antrum, partially occluding the lumen (Figure 2). Biopsy showed an atypical cellular infiltrate of myelocytes, neutrophils, and lymphocytes, with convoluted monocytoid nuclei, which stained positive for CD43, lysozyme, myeloperoxidase, and Leder stain (Figure 3), and negative for pancytokeratin, CD20, and CD3, diagnostic for granulocytic sarcoma.
A repeat bone marrow biopsy showed 18% blasts. The possibility of MDS transforming into leukemia was considered. We offered our patient induction chemotherapy followed by nonmyeloablative stem cell transplant, but she declined this aggressive treatment option and she was started on decitabine (Dacogen). Her GI symptoms gradually improved and her gastric mass remained stable in size on subsequent CT scans.
In September 2007, the woman developed abdominal fullness and decreased appetite. Her symptoms were attributed to even further enlargement of her spleen. She again declined administration of aggressive chemotherapy. Her splenic enlargement was thought be secondary to myeloid cell infiltration and granulocytic sarcoma of the spleen. She was scheduled to receive radiation therapy, 300 cGy in 10 fractions, but she tolerated only 150 cGy because of myelosuppression. Her spleen reduced in size considerably and she had a great deal of symptomatic relief in terms of abdominal fullness. Her appetite also improved.
FIGURE 3
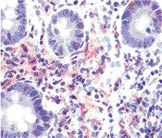
Pathologic Findings-Biopsy showing an atypical cellular infiltrate of myelocytes, neutrophils, and lymphocytes with convoluted monocytoid nuclei, which stained positive for CD43, lysozyme, myeloperoxidase, and Leder stain.
In October 2007, the patient started developing recurrent leukemic pleural effusions on her left side and became symptomatic. Chemotherapy was initiated with doxorubicin and cytarabine, but she died from GI and infectious complications of the chemotherapy during the induction phase.
Discussion
There is no consensus on how to treat granulocytic sarcoma. Generally, these patients are considered as having high-risk AML with a poor outcome, and an early and intensive therapy according to AML protocols is strongly suggested.[10] However, Byrd et al reported that early chemotherapy for MDS-associated GS did not appear to prolong survival. In their collected series, chemotherapy was given to nine patients at the time of initial MDS-associated GS, with a median survival of 36 weeks.[12] External-beam radiation therapy can be used for localized lesions that cause symptoms.
MDS is a disease of the elderly. Aggressive induction chemotherapy in elderly patients is poorly tolerated and results in a higher rate of mortality. Thus, treatment can be even more challenging when an elderly patient presents with granulocytic sarcoma. Our case illustrates the fact that MDS-associated GS can be treated palliatively with radiation and hypomethylating agents in an appropriate setting. With the growing geriatric patient population, effective treatment options are needed in this disease.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Neiman RS, Barcos M, Berard C, et al: Granulocytic sarcoma: A clinicopathologic study of 61 biopsied cases. Cancer 48:1426-1437, 1981.
2. Byrd JC, Edenfield WJ, Shields DJ, et al: Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: A clinical review. J Clin Oncol 13:1800-1816, 1995.
3. Dabbagh V, Browne G, Parapia LA, et al: Granulocytic sarcoma of the rectum: A rare complication of myelodysplasia. J Clin Pathol 52:865-866, 1999.
4. Brugo EA, Marshall RB, Riberi AM, et al: Preleukemic granulocytic sarcomas of the gastrointestinal tract. Report of two cases. Am J Clin Pathol 68: 616-621, 1977.
5. Chennareddy SB, Chennareddy SP, Polidori G, et al: Gastric granulocytic sarcoma as a cause of acute upper gastrointestinal bleeding. Am J Gastroenterology 91:609-611, 1996.
6. Koehler M. Granulocytic sarcoma of the stomach. Gastrointest Endosc 48:190, 1998.
7. Tsimberidou AM, Kantarjian HM, Estey E, et al: Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia 17:1100-1103, 2003.
8. Billstrom R, Johansson B, Fioretos T, et al: Poor survival in t(8,21) (q22:q22)- associated acute myeloid leukemia with leucocytosis. Eur J Haematol 59:47-52, 1997.
9. Homes R, Keating MJ, Cork A, et al: A unique pattern of central nervous system leukemia in acute myelomonocytic leukemia associated with inv (16) (p12;q22). Blood 65:1071-1078, 1985.
10. Breccia M, D´ Andrea M, Morano SG, et al: Granulocytic sarcoma of the pancreas successfully treated with intensive chemotherapy and stem cell transplantation. Eur J Haematol 70:190-192, 2003.
11. List AF, Gonzalez-Osete G, Kummet T, et al: Granulocytic sarcoma in myelodysplastic syndromes: Clinical marker of disease acceleration. Am J Med 90:274-276, 1991.
12. Byrd JC, Edenfield WJ, Dow NS, et al: Extramedullary myeloid cell tumors in myelodysplastic-syndromes: Not a true indication of impending acute myeloid leukemia. Leuk Lymphoma 21:153-159, 1996.