The Heavy Chain Diseases: Clinical and Pathologic Features
This review discusses the clinical presentation; epidemiology; laboratory, radiologic, and pathologic features; and treatment options for each of the heavy chain diseases, emphasising the importance of an accurate pathologic diagnosis and correct interpretation of immunologic studies in their identification.
Figure 1: Immunoglobulin Molecule in Heavy Chain Disease
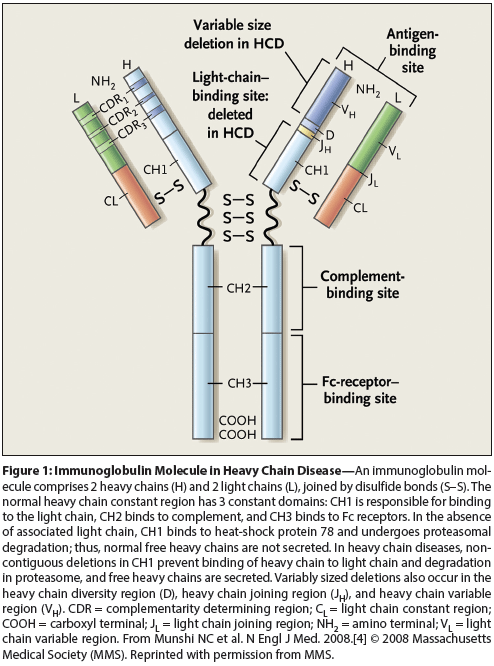
Table: Characteristic Clinicopathologic Features of the Heavy Chain Diseases (HCD)
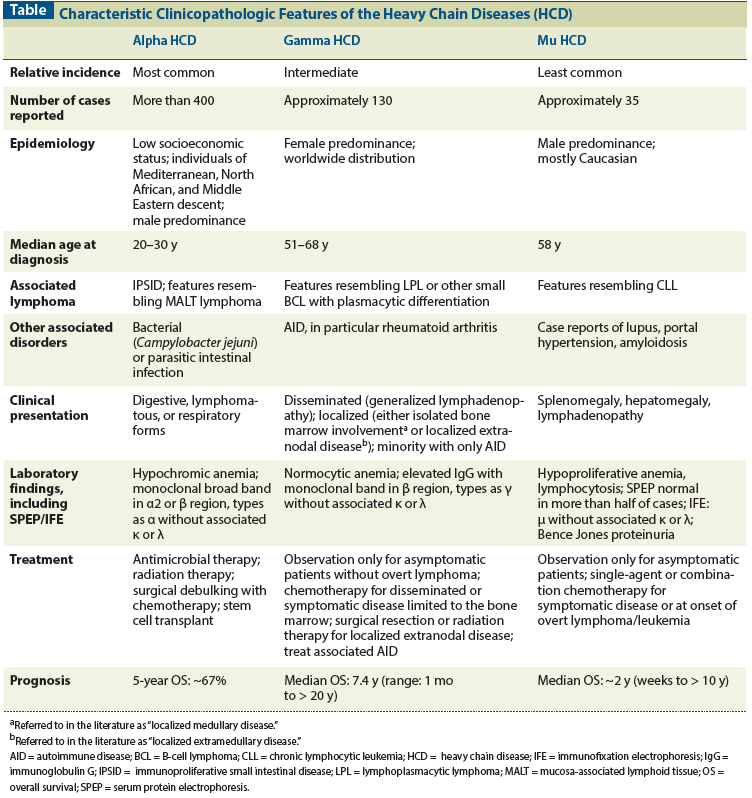
Figure 2: Pathology of Alpha Heavy Chain Disease
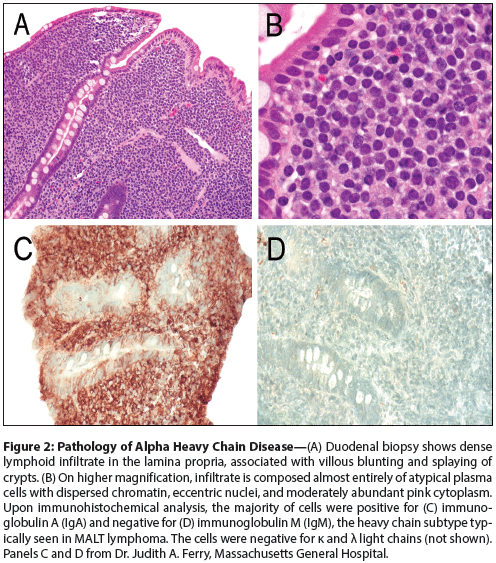
Figure 3: Serum Protein Electrophoresis (SPEP) and Urine Protein Electrophoresis (UPEP) With Immunofixation in Gamma Heavy Chain Disease
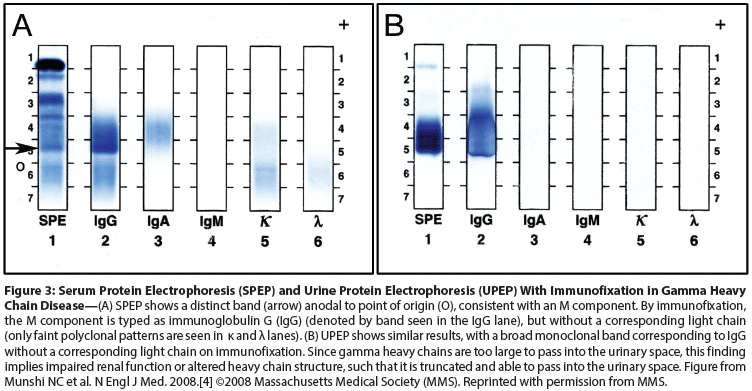
Figure 4: Pathology of Gamma Heavy Chain Disease
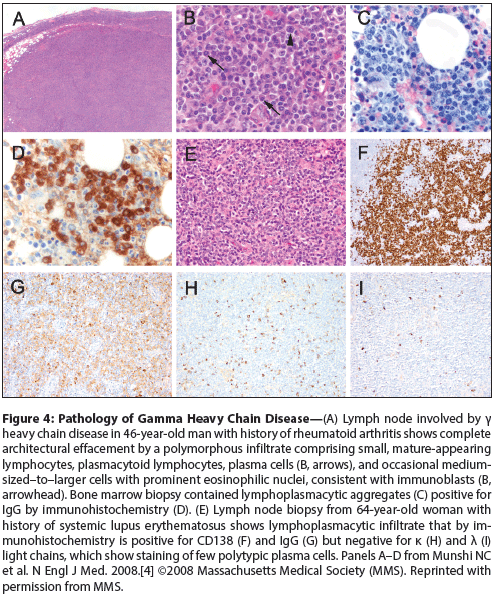
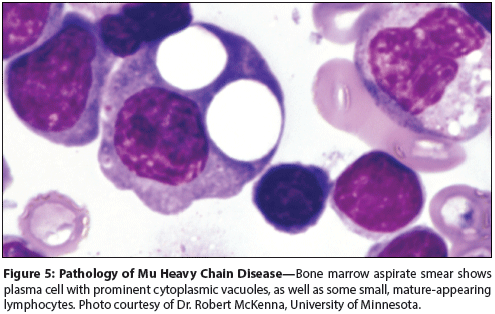
Figure 5: Pathology of Mu Heavy Chain Disease
Heavy chain diseases are a family of rare, systemic syndromes typically associated with or representing a variant of a B-cell neoplasm. Their characteristic feature is production of a mutated immunoglobulin heavy chain incapable of either partnering with light chains in the formation of a full immunoglobulin molecule or of being degraded by the proteasome. The abnormal heavy chain is detected in urine and/or serum without an associated light chain, a pathognomonic finding. Depending on the subtype of the altered heavy chain, these conditions can be subclassified as alpha, gamma, or mu heavy chain disease. We discuss the clinical presentation; epidemiology; laboratory, radiologic, and pathologic features; and treatment options for each of the heavy chain diseases, emphasising the importance of an accurate pathologic diagnosis and correct interpretation of immunologic studies in their identification.
Introduction
The heavy chain diseases are a group of three rare B-cell neoplasms that are clinically and morphologically distinct from one another, but that have in common the production of an abnormal immunoglobulin heavy chain incapable of binding light chains. The altered heavy chains contain deletions, insertions, and point mutations that are acquired during somatic hypermutation. These alterations typically result in loss of a large portion of the constant-1 (CH1) domain of the immunoglobulin heavy chain molecule responsible for light chain binding, with variable effects on the variable (V), diversity (D), and joining (J) regions (Figure 1).[1-3]
In the absence of an associated light chain, the CH1 domain of the normal heavy chain binds to heat-shock protein 78 (hsp78) and undergoes degradation in the proteasome compartment of cells; normal heavy chains unassociated with light chains are therefore never detected in serum or urine. In the heavy chain diseases, the altered structure of the CH1 domain prevents the heavy chain from binding both the light chain and hsp78, thereby allowing it to bypass degradation by the proteasome and be secreted into the serum or urine.[4] In addition, recent work suggests that the altered heavy chain, which forms part of the transmembrane B-cell receptor, may facilitate antigen-independent aggregation and downstream signaling by the receptor, thereby conferring a growth advantage to neoplastic cells.[5]
This common feature gives rise to three different diseases dependent on the heavy chain class that is produced-each with a unique clinical presentation and characteristic findings on immunologic laboratory evaluation and in biopsy specimens of involved tissues. The heavy chain diseases each appear to represent an unusual variant of a type of lymphoma and are not true plasma cell neoplasms.[6] In this review, we describe the clinical and pathologic features of these diseases, with emphasis on clinical presentation and course, and histopathologic and laboratory findings, with a summary of what is known regarding treatment (Table).
Alpha Heavy Chain Disease
Epidemiology and pathogenesis
Alpha (or α) heavy chain disease is the most common of the three heavy chain diseases, with more than 400 cases described in the literature since its initial description in 1968.[7] It has a striking epidemiology, affecting primarily individuals of Mediterranean, Northern African, and Middle Eastern descent, particularly those of low socioeconomic background, suggesting an environmental, possibly infectious, pathogenetic mechanism.[8] Alpha heavy chain disease is most prevalent during the second and third decades of life, with a slight male predominance.
Clinical presentation
Alpha heavy chain disease typically affects the gastrointestinal system; rare cases of respiratory and lymphomatous forms have been reported. The digestive form of alpha heavy chain disease presents as a malabsorption syndrome with weight loss, diarrhea, and abdominal discomfort. Nausea and emesis can also be present. Depending on the degree and duration of the malabsorption, patients can manifest frank growth retardation, amenorrhea, and alopecia. Generalized lymphadenopathy and hepatosplenomegaly are uncommon in the digestive form but are hallmarks of the lymphomatous form, recognized as a distinct entity in 1989.[9] Physical examination in patients with digestive alpha heavy chain disease often reveals ascites and anasarca. Nail clubbing and tetany can also be seen.[10]
Patients with the respiratory form of alpha heavy chain disease can present with dyspnea, mild hypoxemia, and diffuse pulmonary infiltrates, with a restrictive pattern on pulmonary function tests. Hilar lymphadenopathy, lymphomatous involvement of the pharyngeal mucosa, skin rash, and eosinophilia have been reported.[11]
Laboratory features and diagnostic evaluation
Common laboratory abnormalities in patients with alpha heavy chain disease include mild-to-moderate anemia, typically hypochromic; hypoalbuminemia; hypocalcemia; hypokalemia; and hypomagnesemia. Deficiency of lipophilic and hydrophilic vitamins and minerals is frequent. The level of alkaline phosphatase is typically elevated, occasionally to a moderate to severe grade, due to an increase in the gastrointestinal isoform of the enzyme.[10]
Because of defective heavy chain assembly and consequent diversity of immunoglobulin A (IgA) molecular forms, serum protein electrophoresis may appear normal or show hypogammaglobulinemia. When a monoclonal band is identified, it is usually broad and migrates in the α2 or β region of the electrophoretogram, requiring anti-IgA antiserum for its identification by immunofixation.[12] The abnormal alpha heavy chain may be found in jejunal or gastric fluids, but is usually present in only small amounts in urine, and Bence Jones proteinuria has not been described.[12,13]
Microbiologic evaluation for the presence of intestinal bacteria and parasites should be conducted on stool or biopsy specimens. Radiologic studies of the upper gastrointestinal tract can show dilations and strictures of the small bowel loops, hypertrophic or pseudopolypoid mucosa, or coarse mucosal folds. Upper endoscopy is the diagnostic study of choice, since alpha heavy chain disease typically affects the proximal small bowel at the level of the duodenum or jejunum. In a Tunisian-French study conducted in patients with suspected alpha heavy chain disease, five patterns of endoscopic mucosal abnormality were reported. The infiltrative and nodular patterns were the most sensitive and specific for the diagnosis of alpha heavy chain disease; in contrast, ulcerations, mosaic pattern, and isolated mucosal fold thickening alone were nondiagnostic.[14]
Histologic and immunophenotypic characteristics
The lymphoma associated with alpha heavy chain disease typically involves the small intestine and has characteristic features of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma); in this clinicopathologic setting, it has been termed immunoproliferative small intestinal disease (IPSID). The lamina propria of the bowel is heavily infiltrated by a lymphoplasmacytic infiltrate rich in plasma cells, admixed with small lymphocytes resembling marginal zone B cells; lymphoepithelial lesions may also be present (Figure 2).[15-17] The infiltrate separates the crypts, and villous atrophy may be seen.[15,17] The plasma cells and marginal zone cells express monoclonal cytoplasmic alpha chain without light chains. The marginal zone cells express pan-B-cell antigens and are negative for CD5 and CD10, while the plasma cells are positive for CD138 and negative for CD20.[15,17] An association between IPSID and Campylobacter jejuni infection has been identified in some cases, suggesting a causative role for this organism in IPSID pathogenesis, analogous to that of Helicobacter pylori in gastric MALT lymphoma.[17] However, C jejuni has also been detected in other lymphomas of the small intestine, and to date no laboratory studies of the effects of C jejuni on lymphoma cells or in an animal model have been reported.[18,19]
Treatment and prognosis
Given that alpha heavy chain disease has a higher incidence in individuals of lower socioeconomic status, a primary-prevention strategy to improve sanitation would likely have a profound impact on its incidence. Its natural history without treatment is local progression initially, followed by systemic spread. Enlargement of lymphomatous masses typically leads to local complications, including small bowel obstruction, perforation, and intussusception, which can be fatal. Profound malnutrition and cachexia or infectious complications are other causes of death in this population.[20]
Any documented bacterial or parasitic gastrointestinal infection should be eradicated with appropriate antimicrobial therapy. A trial of empiric antibiotic therapy with metronidazole, ampicillin, or tetracycline should be administered, even in the absence of a documented infection, to improve malabsorption symptoms and assess for disease responsiveness. A 6-month course of antimicrobial therapy is the shortest duration shown to be effective treatment, although regression of symptoms is typically observed early in antibiotic-sensitive disease. In patients with early-stage disease, a response rate to antimicrobial treatment between 33% and 71% has been reported associated with documented clinical, laboratory, and histological remission; however, disease recurrence is frequent.[21] Patients should therefore be closely monitored to assess for disease progression while receiving antibiotics. Refractory disease is treated with either total abdominal radiation or, more commonly, combination chemotherapy. There is no standardized regimen for the treatment of alpha heavy chain disease, but doxorubicin-containing combination chemotherapy such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CHVP (cyclophosphamide, doxorubicin, teniposide, and prednisone), or ABV (doxorubicin, bleomycin, and vinblastine) appears to be superior to doxorubicin-free regimens such as COPP (cyclophosphamide, vincristine, procarbazine, and prednisolone); however, the latter may be better tolerated in severely malnourished patients.[22-24] The complete remission rate after treatment with multidrug chemotherapy is 64%, and 5-year overall survival is 67%.[21] Surgical debulking of the tumor mass can be pursued, followed by systemic chemotherapy.[25] For patients with refractory or relapsing disease, high-dose therapy with autologous hematopoietic stem cell transplantation should be considered.
Gamma Heavy Chain Disease
Epidemiology and pathogenesis
Gamma (or γ) heavy chain disease is also called Franklin disease, after the physician who first described it in 1964.[26] It is uncommon, with approximately 130 cases reported in the literature. In recent series, the median age at diagnosis is 51 to 68 years, and there is a clear female predominance.[27,28]
While the pathogenesis of gamma heavy chain disease is unknown, an autoimmune disease is present in about 25% of patients. Rheumatoid arthritis is the most common autoimmune condition associated with gamma heavy chain disease; Sjögren syndrome, systemic lupus erythematosus, vasculitis, and myasthenia gravis, as well as autoimmune cytopenias, including idiopathic thrombocytopenic purpura, have also been reported.[29] Manifestations of autoimmune disease often precede gamma heavy chain disease by many years. Most patients with gamma heavy chain disease (83% to 91%) have an underlying systemic or localized lymphoplasmacytic neoplasm.
Clinical presentation
Gamma heavy chain disease has a heterogeneous clinical and pathologic presentation. Overall, three patterns of disease can be identified, based on the presence or absence of an associated lymphoma. Disseminated lymphoma is seen in 57% to 66% of patients,[8] who typically present with constitutional symptoms, including fever, malaise, and weight loss, which are associated in 50% of cases with generalized lymphadenopathy; splenomegaly; and, less commonly, hepatomegaly.[27] Approximately one-quarter of patients present with lymphoma limited to the bone marrow (referred to in the literature as localized medullary disease) or with localized extranodal disease (referred to in the literature as localized extramedullary disease). The most commonly reported extranodal site of disease is the skin; involvement of the thyroid or parotid gland, the oropharyngeal cavity, and the gastrointestinal tract have been reported.[27,29] Finally, 9% to 17% of patients have no evident lymphoplasmacytic neoplasm at diagnosis; the majority of these patients have a pre-existing autoimmune disease. Clinical manifestations are mainly those of the autoimmune disease, including rheumatoid nodules, rashes, synovitis, and joint deformities.
Laboratory features and diagnostic evaluation
Cytopenias, in particular a normochromic, normocytic anemia, is present at diagnosis due to either an autoimmune disease or bone marrow infiltration. Coombs-positive autoimmune hemolytic anemia and autoimmune thrombocytopenia may be present. Occasionally, circulating monoclonal plasmacytoid lymphocytes or plasma cells are evident, and rarely, features of chronic lymphocytic leukemia or plasma cell leukemia may be present.[8]
The diagnosis of gamma heavy chain disease requires the demonstration of abnormal immunoglobulin protein consisting solely of gamma heavy chain, without associated light chains, by serum or urine immunofixation studies (Figure 3). The abnormal, truncated gamma heavy chain is often present in urine, due to its low molecular weight and existence as a dimer (rather than a polymer, as is the case for alpha and mu heavy chain disease proteins).[2] Persistent absence of light chains on immunofixation after treatment of the serum with 2-mercapto-ethanol, a substance that makes light chains more accessible to antisera by destroying immunoglobulin polymers, can help to substantiate the diagnosis. Supporting laboratory findings include elevation in serum immunoglobulin G (IgG), without accompanying elevation in serum free light chains. Clinical and laboratory distinction of gamma heavy chain disease from an infectious or inflammatory process is sometimes difficult due to the protean clinical presentations, with constitutional symptoms and a history of autoimmune disease seen in a large proportion of patients overall; this complexity is compounded by the tendency of the monoclonal band to migrate in the β region of the electrophoretogram, where it may be obscured by other proteins.[4,27,29] Immunofixation electrophoresis is used to characterize the monoclonal band. Rarely, biclonal gammopathy with an additional intact monoclonal immunoglobulin may be present in the serum, or small amounts of free light chain may be excreted in urine as Bence Jones protein.[27,30]
Histologic and immunophenotypic characteristics
The associated lymphoma typically involves bone marrow, spleen, and lymph nodes, but patients may also present with localized extranodal disease in sites often involved in MALT lymphoma, such as skin, thyroid, salivary glands, gastrointestinal tract, and conjunctiva.[28,29] Examination of involved tissues usually demonstrates a mixed population of lymphocytes, plasmacytoid lymphocytes, and plasma cells that is morphologically similar to lymphoplasmacytic lymphoma (Figure 4). The infiltrate is often more polymorphous, with variable numbers of immunoblasts, eosinophils, and histiocytes; rarely, atypical Reed-Sternberg–like cells suggest a morphologic differential diagnosis of Hodgkin lymphoma or certain types of peripheral T-cell lymphoma.[29] Other cases may demonstrate clinicopathologic features of other small B-cell neoplasms, including MALT lymphoma, splenic marginal zone lymphoma, or other splenic small B-cell neoplasms,[28] illustrating the pathologic heterogeneity of gamma heavy chain disease relative to the alpha and mu subtypes. This histologic diversity, with many cases demonstrating features unlike lymphoplasmacytic lymphoma, suggests gamma heavy chain disease is pathogenetically distinct from lymphoplasmacytic lymphoma; this is further supported by a study of 11 cases of gamma heavy chain disease showing all were negative for MYD88 L265P, a recurrent somatic mutation found in the majority of lymphoplasmacytic lymphomas.[31,32]
The lack of production of immunoglobulin light chains by IgG-positive B cells and plasma cells can be shown by immunohistochemical or in-situ hybridization studies on paraffin-embedded tissue or by flow cytometry (see Figure 4). Importantly, the plasmacytic differentiation will be missed if only kappa and lambda light chain staining is done, as in the usual workup for lymphoma. The neoplastic cells express mature B-cell antigens (CD19, CD20), and lack CD5 and CD10; depending on the extent of plasmacytic differentiation, they may demonstrate partial or complete expression of post–germinal center B-cell and plasma cell markers, such as Mum1/IRF4, CD38, and CD138 (see Figure 4).[4,28]
Treatment and prognosis
Treatment of gamma heavy chain disease is typically tailored to the symptomatology and to the presence of an accompanying autoimmune disease or overt lymphoma. Consequently, there is no standardized treatment. For patients presenting with disseminated lymphoma or with symptomatic disease limited to the bone marrow (localized medullary disease), chemotherapy is recommended. Regimens include chlorambucil; rituximab in CD20-positive disease; melphalan and prednisone, or bortezomib and prednisone, for plasma cell–predominant disease; and CHOP (with rituximab in CD20-positive cases) for aggressive, refractory disease. The combination of fludarabine and rituximab has recently been effective in controlling disease in patients with gamma heavy chain disease and associated pancytopenia.[33] Successful treatment of localized extranodal disease with surgical resection or radiation therapy has also been reported.[29] Patients with no overt lymphoma or who are asymptomatic can be followed expectantly without therapy. Autoimmune disease should be treated according to usual guidelines. Adequate infectious disease prophylaxis and surveillance are critical, particularly in patients receiving immunosuppressive treatments.
In view of the heterogeneity of the disease and treatment, the prognosis is highly variable. Patients with no overt lymphoma may have prolonged survival without treatment, even with occasional spontaneous remissions.[29] Those with treated, localized lymphoma may achieve a sustained complete clinical and immunologic remission. Those with systemic lymphoma may have either an aggressive, rapidly progressive course associated with poor prognosis or may have indolent disease. In the Mayo Clinic series, median survival was 7.4 years (range, 1 month to more than 2 decades).[27] The serum level of monoclonal gamma heavy chain can be used to assess disease evolution and response to treatment.
Mu Heavy Chain Disease
Epidemiology and pathogenesis
In 1970, Dr. Forte, a hematology fellow at Mount Sinai School of Medicine, New York, and Dr. Ballard from the Hematology Department at the New York Veterans Administration Hospital, independently reported the first two cases of mu (or μ) heavy chain disease.[34,35] Both patients were men in their late fifties, presenting with unremitting joint pain or stiffness. Mu heavy chain disease is the rarest in this family of conditions, with only 30 to 40 cases reported in the literature. The disease occurs predominantly in Caucasian men, with a median age of 58 years at diagnosis.[8] The cause of mu heavy chain disease is not known, but most patients have a lymphoid neoplasm resembling chronic lymphocytic leukemia/small lymphocytic lymphoma.[1]
Clinical presentation
The symptoms and signs of mu heavy chain disease are related to the associated lymphoma. Splenomegaly is almost universally present, with hepatomegaly in three-quarters of patients.[8] Palpable, superficial lymphadenopathy is identified in 40% of patients. The literature contains single case reports of mu heavy chain disease associated with systemic lupus erythematosus, portal hypertension, recurrent pulmonary infections, splenomegaly, and pancytopenia, as well as myelodysplastic syndrome and systemic amyloidosis, each with clinical features reflecting the accompanying disorder.[36,37] In one case, diffuse large B-cell lymphoma of the breast was diagnosed 2 years after successful initial treatment with single-agent cyclophosphamide.[38] Inter-estingly, the original 1970 papers by Drs. Forte and Ballard each reported pathologic lytic bone lesions that were attributed to lymphocytic infiltration of bone marrow. Lytic bone lesions have been subsequently reported in about 20% of patients. Carpal tunnel syndrome and amyloid deposition in the rectal mucosa were also present in the first two patients with mu heavy chain disease.[39]
Laboratory features and diagnostic evaluation
The most common laboratory abnormality is anemia, typically hypoproliferative and related to bone marrow infiltration by neoplastic cells. Thrombocytopenia is less common, and lymphocytosis may be present. Routine serum protein electrophoresis is normal in more than half of cases.[1] Immunofixation reveals reactivity to anti-mu in polymers of diverse sizes, with no associated kappa or lambda light chain.[1,36,37,40,41] In a few cases, biclonal gammopathy with an additional intact monoclonal immunoglobulin may be present.[1] Although mu heavy chain is only rarely found in the urine, the neoplastic cells also produce monoclonal light chains, usually of kappa type, that fail to assemble with the truncated heavy chain and are excreted in the urine as Bence Jones protein.[1,35,42] Bence Jones proteinuria, although frequently detected, rarely causes renal complications.[42]
Cytologic and immunophenotypic characteristics
Bone marrow aspirate smears and touch preparations contain characteristic plasma cells with prominent cytoplasmic vacuoles, which are typically admixed with small, round lymphocytes resembling those seen in chronic lymphocytic leukemia (Figure 5).[1,35,37] Results of immunophenotypic analysis have been reported in only a few cases: the neoplastic cells express cytoplasmic immunoglobulin M (IgM) and lack light chain staining by immunohistochemistry; by flow cytometry, they express CD19, CD20, and CD38.[38,43] Weak expression for kappa light chain or CD5 has been reported rarely.[1,38]
Treatment and prognosis
Data regarding treatment and prognosis are limited because of the rarity of this disease. Detectable monoclonal mu heavy chain in a patient who is otherwise asymptomatic requires only observation without therapy.[44] If and when an underlying malignancy develops, treatment regimens reported in the literature include CHOP, CVP (cyclophosphamide, vincristine, and prednisone), single-agent fludarabine, and single-agent cyclophosphamide.[8,43] Median overall survival is approximately 2 years, with a large range-from less than a month to over a decade. However, these statistics are likely underestimates of overall survival, since the presence of monoclonal mu heavy chains is frequently missed on serum protein electrophoresis, especially in the absence of an associated overt lymphoma. A spontaneous remission of mu heavy chain disease has been reported.[8]
Conclusion
Heavy chain diseases are a family of rare disorders characterized by alterations in the immunoglobulin heavy chain resulting in the pathognomonic finding of free heavy chains without associated light chains in the serum and/or urine. Diagnosis of these diseases remains challenging due to their rarity; their nonspecific clinical presentations; and the skill required in interpreting immunologic laboratory tests and tissue biopsies from affected patients, necessitating close collaboration between clinicians and pathologists. In addition, it remains difficult to outline the best treatment approaches for these uncommon conditions. The advent of molecular genetic techniques in lymphoma diagnosis may pave the way for improved diagnostic techniques and treatment approaches, as well as a better understanding of the pathogenesis underlying this fascinating group of diseases.
Acknowledgments:The authors are grateful to Dr. Mandakolathur R. Murali for his critical review of the manuscript, and to Dr. Judith A. Ferry, Michelle Forrestall Lee, and Stephen Conley for their assistance with figure composition.
Financial Disclosure:Dr. Anderson serves on the advisory boards of Celgene, Gilead, Novartis, Onyx, and Sanofi-Aventis. The remaining authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Wahner-Roedler DL, Kyle RA. Mu-heavy chain disease: presentation as a benign monoclonal gammopathy. Am J Hematol. 1992;40:56-60.
2. Fermand JP, Brouet JC. Heavy-chain diseases. Hematol Oncol Clin North Am. 1999;13:1281-94.
3. Goossens T, Klein U, Kuppers R. Frequent occurrence of deletions and duplications during somatic hypermutation: implications for oncogene translocations and heavy chain disease. Proc Natl Acad Sci U S A. 1998;95:2463-8.
4. Munshi NC, Digumarthy S, Rahemtullah A. Case records of the Massachusetts General Hospital. Case 13-2008. A 46-year-old man with rheumatoid arthritis and lymphadenopathy. N Engl J Med. 2008;358:1838-48.
5. Corcos D, Osborn MJ, Matheson LS. B-cell receptors and heavy chain diseases: guilty by association? Blood. 2011;117:6991-8.
6. Harris NL, Isaacson PG, Grogan TM, Jaffe ES. Heavy chain diseases. In: Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of the haematopoietic and lymphoid tissues. Lyon: IARC; 2008. p. 196-9.
7. Seligmann M, Danon F, Hurez D, et al. Alpha-chain disease: a new immunoglobulin abnormality. Science. 1968;162:1396-7.
8. Wahner-Roedler DL, Kyle RA. Heavy chain diseases. Best Pract Res Clin Haematol. 2005;18:729-46.
9. Takahashi K, Naito M, Matsuoka Y, Takatsuki K. A new form of alpha-chain disease with generalized lymph node involvement. Pathol Res Pract. 1988;183:717-23.
10. Doe WF, Henry K, Hobbs JR, et al. Five cases of alpha chain disease. Gut. 1972;13:947-57.
11. Stoop JW, Ballieux RE, Hijmans W, Zegers BJ. Alpha-chain disease with involvement of the respiratory tract in a Dutch child. Clin Exp Immunol. 1971;9:625-35.
12. Seligmann M. Alpha chain disease: immunoglobulin abnormalities, pathogenesis and current concepts. Br J Cancer Suppl. 1975;2:356-61.
13. Al-Saleem T, Al-Mondhiry H. Immunoproliferative small intestinal disease (IPSID): a model for mature B-cell neoplasms. Blood. 2005;105:2274-80.
14. Halphen M, Najjar T, Jaafoura H, et al. Diagnostic value of upper intestinal fiber endoscopy in primary small intestinal lymphoma. A prospective study by the Tunisian-French Intestinal Lymphoma Group. Cancer. 1986;58:2140-5.
15. Isaacson PG, Dogan A, Price SK, Spencer J. Immunoproliferative small-intestinal disease. An immunohistochemical study. Am J Surg Pathol. 1989;13:1023-33.
16. Parsonnet J, Isaacson PG. Bacterial infection and MALT lymphoma. N Engl J Med. 2004;350:213-5.
17. Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med. 2004;350:239-48.
18. Peterson MC. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med. 2004;350:1685-6.
19. Isaacson PG. Extranodal marginal zone lymphoma: MALT lymphoma. In: Jaffe ES, Harris NL, Vardiman JW, et al, editors. Hematopathology. St. Louis: Elsevier; 2011. p. 291-305.
20. Alpha-chain disease and related small-intestinal lymphoma: a memorandum. Bull World Health Organ. 1976;54:615-24.
21. Fine KD, Stone MJ. Alpha-heavy chain disease, Mediterranean lymphoma, and immunoproliferative small intestinal disease: a review of clinicopathological features, pathogenesis, and differential diagnosis. Am J Gastroenterol. 1999;94:1139-52.
22. Ben-Ayed F, Halphen M, Najjar T, et al. Treatment of alpha chain disease. Results of a prospective study in 21 Tunisian patients by the Tunisian-French intestinal Lymphoma Study Group. Cancer. 1989;63:1251-6.
23. Salem PA, Estephan FF. Immunoproliferative small intestinal disease: current concepts. Cancer J. 2005;11:374-82.
24. Akbulut H, Soykan I, Yakaryilmaz F, et al. Five-year results of the treatment of 23 patients with immunoproliferative small intestinal disease: a Turkish experience. Cancer. 1997;80:8-14.
25. Martin IG, Aldoori MI. Immunoproliferative small intestinal disease: Mediterranean lymphoma and alpha heavy chain disease. Br J Surg. 1994;81:20-4.
26. Franklin EC, Lowenstein J, Bigelow B, Meltzer M. Heavy chain disease-a new disorder of serum gamma-globulins: report of the first case. Am J Med. 1964;37:332-50.
27. Wahner-Roedler DL, Witzig TE, Loehrer LL, Kyle RA. Gamma-heavy chain disease: review of 23 cases. Medicine (Baltimore). 2003;82:236-50.
28. Bieliauskas S, Tubbs RR, Bacon CM, et al. Gamma heavy-chain disease: defining the spectrum of associated lymphoproliferative disorders through analysis of 13 cases. Am J Surg Pathol. 2012;36:534-43.
29. Fermand JP, Brouet JC, Danon F, Seligmann M. Gamma heavy chain “disease”: heterogeneity of the clinicopathologic features. Report of 16 cases and review of the literature. Medicine (Baltimore). 1989;68:321-35.
30. Presti BC, Sciotto CG, Marsh SG. Lymphocytic lymphoma with associated gamma heavy chain and IgM-lambda paraproteins. An unusual biclonal gammopathy. Am J Clin Pathol. 1990;93:137-41.
31. Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. 2012;367:826-33.
32. Hamadeh F, MacNamara S, Bacon CM, et al. Gamma heavy chain disease lacks the MYD88 L265P mutation associated with lymphoplasmacytic lymphoma (abstract). United States and Canadian Academy of Pathology; San Diego, Calif. 2014.
33. Inoue D, Matsushita A, Kiuchi M, et al. Successful treatment of gamma-heavy-chain disease with rituximab and fludarabine. Acta Haematol. 2012;128:139-43.
34. Forte FA, Prelli F, Yount WJ, et al. Heavy chain disease of the gamma (gamma M) type: report of the first case. Blood. 1970;36:137-44.
35. Ballard HS, Hamilton LM, Marcus AJ, Illes CH. A new variant of heavy-chain disease (mu-chain disease). N Engl J Med. 1970;282:1060-2.
36. Witzens M, Egerer G, Stahl D, et al. A case of mu heavy-chain disease associated with hyperglobulinemia, anemia, and a positive Coombs test. Ann Hematol. 1998;77:231-4.
37. Iwasaki T, Hamano T, Kobayashi K, Kakishita E. A case of mu-heavy chain disease: combined features of mu-chain disease and macroglobulinemia. Int J Hematol. 1997;66:359-65.
38. Maeda A, Mori M, Torii S, et al. Multiple extranodal tumors in mu-heavy chain disease. Int J Hematol. 2006;84:286-7.
39. Kinoshita K, Yamagata T, Nozaki Y, et al. Mu-heavy chain disease associated with systemic amyloidosis. Hematology. 2004;9:135-7.
40. Maisnar V, Tichy M, Stulik J, et al. Capillary immunotyping electrophoresis and high resolution two-dimensional electrophoresis for the detection of mu-heavy chain disease. Clin Chim Acta. 2008;389:171-3.
41. Tamura A, Yamashiro A, Mizutani F, et al. Immunochemical properties of free mu-chain protein in a patient with mu-heavy chain disease [Japanese]. Rinsho Byori. 2003;51:847-51.
42. Preud’homme JL, Bauwens M, Dumont G, et al. Cast nephropathy in mu heavy chain disease. Clin Nephrol. 1997;48:118-21.
43. Yanai M, Maeda A, Watanabe N, et al. Successful treatment of mu-heavy chain disease with fludarabine monophosphate: a case report. Int J Hematol. 2004;79:174-7.
44. Witzig TE, Wahner-Roedler DL. Heavy chain disease. Curr Treat Options Oncol. 2002;3:247-54.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.