HER2-Positive Breast Cancer: Beyond Trastuzumab
This review explores the use of several such agents, including lapatinib (Tykerb), HSP90 inhibitors, T-DM1, and other tyrosine kinase inhibitors. Emerging data from trials of these agents indicate that the HER2 pathway remains a valid therapeutic target following disease progression on trastuzumab.
The outlook for patients with HER2-positive breast cancer was revolutionized by the development of trastuzumab (Herceptin), a humanized murine monoclonal antibody. Use of this agent led to improved overall survival when it was added to chemotherapy for the treatment of metastatic breast cancer. Improved understanding of mechanisms of resistance to trastuzumab has facilitated the development of novel agents for HER2-positive breast cancer, and also resulted in superior outcomes when added to chemotherapy in the adjuvant setting. This review explores the use of several such agents, including lapatinib (Tykerb), HSP90 inhibitors, T-DM1, and other tyrosine kinase inhibitors. Emerging data from trials of these agents indicate that the HER2 pathway remains a valid therapeutic target following disease progression on trastuzumab, and suggest a promising role for combined HER2 blockade with two or more agents.
The human epidermal growth factor receptor 2 (HER2) gene encodes a transmembrane glycoprotein receptor belonging to a family of growth factor receptors (the ErbB or HER family) with intrinsic tyrosine kinase activity. The initial descriptions of HER2 focused on the negative prognostic implications of amplification of the gene and/or overexpression of its product.1,2 The outlook for patients with HER2-positive breast cancer was revolutionized by the development of trastuzumab (Herceptin), a humanized murine monoclonal antibody targeting an extracellular domain (domain IV) of the receptor.3 This antibody led to improved outcomes, including overall survival, when added to chemotherapy for the treatment of metastatic breast cancer.4 It was subsequently explored in several large adjuvant studies, in which the addition of trastuzumab to chemotherapy for early breast cancer was found to reduce the risk of recurrence and death.5 The results of these studies have led to the incorporation of trastuzumab as an integral component of chemotherapy regimens for early and advanced breast cancer.
Despite the major advance in the therapy of HER2-positive breast cancer represented by this agent, nearly all patients treated with trastuzumab for advanced breast cancer will ultimately experience progression, while a significant proportion of patients receiving this treatment in the setting of early breast cancer will experience disease recurrence. Some may receive further trastuzumab in the metastatic setting, but again, will likely experience progression despite this treatment. Accordingly, a large volume of research has centered on identifying mechanisms of resistance to trastuzumab, and developing novel agents to target the aberrant growth signaling pathway represented by HER2. Key components of the HER2 pathway are illustrated in Figure 1, along with the sites of action of novel agents targeting this pathway.
Mechanisms of Resistance
FIGURE 1
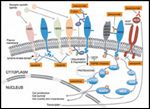
Sites of Action of the Agents Discussed Along the HER2-Signaling Pathway
Potential mechanisms underlying trastuzumab resistance include blocking of the interaction of trastuzumab with the HER2 receptor, interaction of the receptor with other growth factor receptors, and downstream activation of signaling pathways. Trastuzumab binding may be physically prevented by increased expression of the membrane glycoprotein MUC46 or by shedding of the extracellular domain of the receptor containing the trastuzumab-binding site.7 Crosstalk or heterodimerization between HER2 and IGF-1R or other HER family members may be a mechanism of resistance to trastuzumab, allowing activation of interlinked signaling pathways and resulting in cell proliferation.6,8 Trastuzumab's inhibition of HER2 signaling along the PI3K/AKT pathway may be bypassed by activating mutations of AKT and decreased expression of PTEN, a negative regulator of this pathway.9,10 Another potential mechanism of resistance involves transcriptional upregulation of HER2 gene expression.11
Tyrosine Kinase Inhibitors: Lapatinib
By targeting the intracellular tyrosine kinase moiety of HER2, mechanisms of resistance affecting the extracellular component (including increased glycoprotein expression, shedding of the extracellular domain, etc) may be countered. Lapatinib (Tykerb) was the first such agent to be approved for HER2-positive breast cancer. This is an orally available, reversible small molecule tyrosine kinase inhibitor (TKI) that targets not only HER2 but also the epidermal growth factor receptor (HER1/EGFR). Results for this agent as monotherapy in heavily pretreated patients with HER2-positive metastatic breast carcinoma were disappointing, with response rates of 5% or less.12,13 In the first-line setting, lapatinib monotherapy yielded a more promising overall response rate of 24%,14 which is comparable to the outcome for trastuzumab monotherapy in the first-line setting.15
Lapatinib Plus Chemotherapy
EGF100151 was an open-label phase III study that investigated the addition of lapatinib to capecitabine (Xeloda) in patients with HER2-positive advanced breast cancer previously treated with an anthracycline, a taxane, and trastuzumab.16 The control arm consisted of capecitabine at 2,500 mg/m2 daily for 14 out of every 21 days, which is the US Food and Drug Administration (FDA)-approved dosing regimen. The investigational arm received lapatinib at 1,250 mg daily continuously with a lower dose of capecitabine (2,000 mg/m2 daily). Study accrual was discontinued after a prespecified event analysis on 324 patients demonstrated a significant improvement in time to progression for the combination treatment (hazard ratio [HR] = 0.49; 95% confidence interval [CI] = 0.34–0.71; P < .001).
Updated analysis of the total accrued population of 399 patients confirmed a significant improvement in time to progression and overall response rate with the combined treatment. There was no difference in survival. The most common toxicity for the combination was diarrhea (occurring in 60%, with G3 in 12% and grade 4 in 1%). A second upfront phase III study randomized 579 patients with advanced breast cancer to lapatinib (1,500 mg daily) or placebo in addition to paclitaxel every 3 weeks.17 No patients with known HER2-positive disease were enrolled, although central HER2 testing was performed as part of the study. This revealed 86 patients with HER2-positive disease. In this subgroup of patients, time to progression, event-free survival, overall response rate, and clinical benefit rate were all significantly improved with the combination, whereas no improvement in any of the efficacy endpoints was seen in the HER2-negative population.
Toxicities seen more commonly with the combination included rash, diarrhea, sepsis, and vomiting. In addition, there was an increased rate of fatal adverse events in the combination arm (2.7% vs 0.6%), including three cases of sepsis related to diarrhea. This highlights the importance of early and effective antidiarrheal management in patients treated with lapatinib, as well as the potential of other agents to potentiate this toxicity.
Continuing Trastuzumab
In the years following the incorporation of trastuzumab into the routine treatment of HER-positive metastatic breast cancer, a prevailing debate has centered on the appropriateness of continuing trastuzumab in the setting of disease progression. The experience with conventional cytotoxic agents suggests that it is futile to continue therapy beyond progression, but whether this dictum applied to novel biologic agents was unknown. In practice, many oncologists continued trastuzumab into second and greater lines of therapy, while changing the chemotherapy partner drug. This practice was not based on any prospective evidence, however.
Two recent randomized studies have finally answered this debate. In the first, patients progressing after trastuzumab-based therapy were randomized to capecitabine with and without trastuzumab.18 Accrual was halted early after 156 of 482 patients were enrolled, when a preplanned interim analysis indicated a significant improvement in progression-free survival (PFS) from 5.6 to 8.2 months (P = .03) in the patients continuing trastuzumab.
A second study investigated the role of continuing trastuzumab in the setting of lapatinib monotherapy.19,20 A total of 296 patients who had received a median of three prior trastuzumab-containing regimens for HER2-positive metastatic breast cancer were randomized to lapatinib at 1,500 mg daily or lapatinib at 1,000 mg daily plus weekly trastuzumab treatment. Despite a built-in crossover to the combination for patients progressing on lapatinib monotherapy, a significant improvement in overall survival was demonstrated with the combination therapy (HR = 0.74; 95% CI = 0.57–0.97; P = .026). Continued trastuzumab led to an improvement in PFS and clinical benefit rate, with a trend for improved overall survival despite a built-in crossover to the combination for patients progressing on lapatinib monotherapy (HR = 0.75; 95% CI = 0.53–1.07; P = .106). As well as validating the common practice of continuing trastuzumab after progression, this study confirmed a role for combined HER2 blockade, which is a feature of many current studies evaluating novel HER2-targeting agents.
HSP90 Inhibitors
Heat shock protein 90 (HSP90) belongs to a class of molecular chaperones whose intracellular function involves facilitating the maturation and stability of many client proteins, including HER2 as well as the protein products of other oncogenes.21 Inhibition of HSP90 ultimately leads to the degradation of client proteins in the proteasome. A phase I study evaluated the combination of trastuzumab with the HSP90 inhibitor tanespimycin (a derivative of the antibiotic geldanamycin).22 Escalating doses of weekly intravenous tanespimycin were combined with conventional weekly trastuzumab in 25 patients with advanced solid tumors, including 15 with HER2-positive metastatic breast cancer. One partial response and four tumor regressions of between 21% and 35% were seen among 15 patients with metastatic breast cancer.
The combination was then evaluated in a single-arm phase II study in 31 patients with metastatic breast cancer following one prior line of trastuzumab-containing therapy.23 Of 27 patients eligible for response assessment, 7 experienced partial responses (26%). An additional 5 patients experienced disease regressions of between 20% and 29%, and 5 patients had disease stabilization for more than 4 months, for a clinical benefit rate of 63%. Several studies are currently assessing HSP90 inhibitors for advanced HER2-positive breast cancer, including a multicenter phase II study evaluating the combination of trastuzumab and IPI-504, an agent that interconverts with tanespimycin in vivo.
T-DM1
In recent years the potential of harnessing potent cytotoxic drugs to targeted monoclonal antibodies has been evaluated in several cancer types. The attraction of this approach is that the cytotoxic effect of the chemotherapy agent may be directly delivered to tumor cells, while potentially reducing harmful side effects to normal cells. The first such antibody drug conjugate (ADC) to be explored in breast cancer is T-DM1, a drug created by linking a derivative of the antimicrotubule agent maytansine to trastuzumab. T-DM1 given intravenously every 3 weeks at 3.6 mg/kg was evaluated in a single-arm phase II study in 112 patients with previously treated HER2-positive metastatic breast cancer.24 This study demonstrated an overall response rate of 26.9%, with a similar overall response rate of 24.2% among patients previously treated with both trastuzumab and lapatinib.
A second open-label phase II study enrolled 110 patients who had all received prior anthracycline, taxane, capecitabine, trastuzumab, and lapatinib to receive every-3-week TDM1.25 The overall response rate in this population was 32.7% by independent radiology assessment. The most commonly encountered toxicities in these studies were fatigue, nausea, and reversible thrombocytopenia. Ongoing studies are evaluating the use of T-DM1 vs combinations of cytotoxics and HER2-targeted agents in the firstline treatment of advanced disease.
Other TKIs
Neratinib, like lapatinib, is an orally available pan-ErbB TKI, but it differs in that it inhibits HER4 as well as HER1/EGFR and HER2, and it does so in an irreversible manner. A phase I study in advanced solid tumors established the maximum tolerated dose of 320 mg daily.26 The most common adverse events with this agent included diarrhea, nausea, fatigue, and vomiting, and the dose-limiting toxicity was grade 3 diarrhea. A phase II study in breast cancer utilized a lower dose of 240 mg daily (owing to gastrointestinal effects seen in another study at the 320-mg daily dose), with a response rate of 26% in patients who had previously received trastuzumab, and 56% in patients who had not received prior trastuzumab.27
Neratinib was subsequently tested in combination with weekly trastuzumab in a phase I/II study.28 The primary endpoint of the phase II component of this study was to detect an increase in the 16-week progression-free survival from 15% to 35% with the combination. The study met this endpoint with a 16-week progression-free survival of 45%, and an overall response rate of 29%. The most common adverse events were diarrhea, nausea, anorexia, and vomiting, with grade 3 diarrhea in 11% of patients treated with the combination of neratinib at 240 mg daily and trastuzumab in the phase II portion of the study. Safety and efficacy data for neratinib in combination with chemotherapy agents including paclitaxel, capecitabine, and vinorelbine have been presented.29-31
Pertuzumab
Pertuzumab (Omnitarg), like trastuzumab, is a monoclonal HER2-targeted antibody, but it is directed against a different epitope on the receptor (extracellular domain II). Binding of the antibody to this site prevents receptor homo- and heterodimerization, a key step in the passage of downstream growth factor signaling in this receptor group. The combination of pertuzumab with trastuzumab led to an overall response rate of 24.2% in a phase II study in patients with metastatic breast cancer that had progressed after prior trastuzumab.32 An additional cohort, which was later added to this study, received pertuzumab monotherapy, with the option of again adding trastuzumab after disease progression on pertuzumab alone.33 The response rate for pertuzumab alone was low (3.4%). Nevertheless, the overall response rate among 14 patients receiving combination trastuzumab and pertuzumab after progressing on both drugs as single agents was promising (21.4%). These results confirm the validity of combined HER2 blockade to overcome mechanisms of resistance in patients progressing after trastuzumab monotherapy.
Inhibitors of the PI3K/Akt Pathway
In vivo assessment of biopsy samples from patients progressing after trastuzumab therapy demonstrates activation of the PI3K/Akt pathway as a common mechanism underlying trastuzumab resistance.34 Mammalian target of rapamycin (mTOR) is a serine-threonine kinase that is a downstream component of the PI3K/ Akt pathway. Phase I combinations of the oral mTOR inhibitor everolimus (Afinitor) with weekly trastuzumab and chemotherapy in patients with HER2-positive metastatic breast cancer progressing after trastuzumab therapy yielded response rates of 41% with paclitaxel and 18% with vinorelbine.35,36 The most common doselimiting toxicities were neutropenia and stomatitis. Phase II studies are awaited. Inhibitors of PI3K are also being evaluated as potential modulators of trastuzumab sensitivity.
Antiangiogenesis Agents
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Bevacizumab (Avastin)
Capecitabine (Xeloda)
Everolimus (Afinitor)
Geldanamycin
IPI-504
Lapatinib (Tykerb)
Neratinib
Paclitaxel
Pazopanib (Votrient)
Pertuzumab (Omnitarg)
Sorafenib (Nexavar)
Sunitinib (Sutent)
Tanespimycin
T-DM1
Trastuzumab (Herceptin)
Vinorelbine
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Preclinical data demonstrate HER2 and vascular endothelial growth factor (VEGF) pathway interactions, providing a rationale for targeting both receptor pathways in HER2-positive breast cancer.37 A phase II study evaluated the combination of weekly trastuzumab and biweekly bevacizumab (Avastin) as first-line therapy of HER2-positive advanced breast cancer.38 The combined monoclonal antibody treatment achieved an overall response rate of 48% and clinical benefit rate of 60%. The omission of conventional cytotoxic agents does not necessarily make this a "benign" regimen, however: While only 1 of the 50 patients experienced grade 4 cardiotoxicity, an additional 15 patients had asymptomatic declines in left-ventricular ejection fraction (LVEF) and 36% experienced grade 3 hypertension.
The use of combined HER2/VEGF blockade with dual monoclonal antibody therapy in addition to chemotherapy is currently being investigated in the adjuvant setting. Dual tyrosine kinase inhibition of HER2 and VEGF pathways (using lapatinib and pazopanib [Votrient]) in first-line therapy of advanced HER2-positive breast cancer improved the primary endpoint of progressive disease rate at week 12 and yielded marginal improvement in response rates, albeit with increased toxicities including diarrhea, transaminitis, hypertension, and changes in hair color.39 Of 76 patients on the combination arm, 4 experienced cardiac toxicity (asymptomatic LVEF decline ≥ 20% and below the lower limit of normal in 3 patients, and symptomatic LVEF decline < 20% in 1).
The combination of lapatinib (1,500 mg daily) and biweekly bevacizumab was assessed in a more pretreated HER2-positive patient population in a phase II study.40 Patients had received a median of four prior chemotherapy regimens. The confirmed overall response rate among 52 patients was 13.3%. Common toxicities included diarrhea, rash, fatigue, bleeding, nausea, and headache. Three patients-all of whom were asymptomatic-experienced LVEF declines below 50%. Several other agents targeting the VEGF pathway via tyrosine kinase inhibition (eg, sunitinib [Sutent], sorafenib [Nexavar]) or other mechanisms are being evaluated in HER2-positive breast cancer in early-phase clinical studies. Combined HER2/VEGF blockade remains investigational at this time.
Discussion
Improved understanding of mechanisms of resistance to trastuzumab has facilitated the development of novel agents for HER2-positive breast cancer. Emerging data from trials of these agents indicate that the HER2 pathway remains a valid therapeutic target following progression on trastuzumab, and suggest a promising role for combined HER2 blockade with two or more agents. In the coming years, it is anticipated that one or more of these agents will enter routine clinical practice for advanced HER2-positive breast cancer, and may supplement or even replace trastuzumab as the backbone of adjuvant therapy for this unique disease subset.
References:
REFERENCES:1. Slamon DJ, Clark GM, Wong SG, et al: Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177-182, 1987.
2. Tandon AK, Clark GM, Chamness GC, et al: HER2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol 7:1120-1128, 1989.
3. Carter P, Presta L, Gorman CM, et al: Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA 89:4285-4289, 1992.
4. Slamon DJ, Leyland-Jones B, Shak S, et al: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001.
5. Murphy CG, Modi S: HER2 breast cancer therapies: A review. Biologics 3:289-301, 2009.
6. Nahta R, Esteva FJ: HER2 therapy: Molecular mechanisms of trastuzumab resistance. Breast Cancer Res 8(6):215, 2006.
7. Scaltriti M, Rojo F, Ocana A, et al: Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 99:628-638, 2007.
8. Ritter CA, Perez-Torres M, Rinehart C, et al: Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 13:4909-4919, 2007.
9. Nagata Y, Lan KH, Zhou X, et al: PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6:117-127, 2004.
10. Clark AS, West K, Streicher S, et al: Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther 1:707-717, 2002.
11. Vazquez-Martin A, Colomer R, Brunet J, et al: Pharmacological blockade of fatty acid synthase (FASN) reverses acquired autoresistance to trastuzumab (Herceptin by transcriptionally inhibiting 'HER2 super-expression' occurring in high-dose trastuzumab-conditioned SKBR3/Tzb100 breast cancer cells. Int J Oncol 31:769-776, 2007.
12. Burstein HJ, Storniolo AM, Franco S, et al: A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol 19:1068-1074, 2008.
13. Blackwell KL, Pegram MD, Tan-Chiu E, et al: Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol 20:1026-31, 2009.
14. Gomez HL, Doval DC, Chavez MA, et al: Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 26:2999-3005, 2008.
15. Vogel CL, Cobleigh MA, Tripathy D, et al: Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20:719-726, 2002.
16. Cameron D, Casey M, Press M, et al: A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat 112:533-543, 2008.
17. Di Leo A, Gomez HL, Aziz Z, et al: Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol 26:5544-5552, 2008.
18. Von Minckiwitz G, du Bois A, Schmidt M, et al: Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03-05 study. J Clin Oncol 27:1999-2006, 2009.
19. O'Shaughnessy J, Blackwell KL, Burstein H, et al: A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy (abstract 1015). J Clin Oncol 26(15S):44s, 2008.
20. Blackwell KL, Burstein HJ, Storniolo AM, et al: Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28:1124-1130, 2010.
21. Solit DB, Rosen N: Hsp90: A novel target for cancer therapy. Curr Top Med Chem 6:1205-1214, 2006.
22. Modi S, Stopeck AT, Gordon MS, et al: Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: A phase I dose-escalation study. J Clin Oncol 25:5410-5417, 2007.
23. Modi S, Sugarman S, Stopeck A, et al: Phase II trial of the Hsp90 inhibitor tanespimycin (Tan) + trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) (abstract 1027). J Clin Oncol 26(15S):47s, 2008.
24. Vogel CL, Burris HA, Limentani S, et al: A phase II study of trastuzumab-DM1 (TDM1), a HER2 antibody-drug conjugate (ADC), in patients (pts) with HER2+ metastatic breast cancer (MBC): Final results (abstract 1017). J Clin Oncol 27(15S):45s, 2009.
25. Krop I, LoRusso P, Miller KD: A phase II study of trastuzumab-DM1 (T-DM1), a novel HER2 antibody-drug conjugate, in patients previously treated with lapatinib, trastuzumab, and chemotherapy (abstract 5090). Cancer Res 69(24 suppl), 2009.
26. Wong KK, Fracasso PM, Bukowski RM: A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res 15:2552-2558, 2009.
27. Burstein HJ, Sun Y, Dirix LY, et al: Neratinib (HKI-272), an irreversible pan erbB receptor tyrosine kinase inhibitor in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 28:1301-1307, 2010.
28. Burstein HJ, Sun Y, Tan AR, et al: Neratinib (HKI-272), an irreversible pan erbB receptor tyrosine kinase inhibitor: Phase 2 results in patients with advanced HER2+ breast cancer (abstract 37). Cancer Res 69(24 suppl):73s, 2009.
29. Chow L, Gupta S, Hershman D, et al: Safety and efficacy of neratinib (HKI-272) in combination with paclitaxel in ErbB2+ metastatic breast cancer (abstract 5081). Cancer Res 69(24 suppl), 2009.
30. Saura C, Martin M, Moroose R, et al: Safety of neratinib (HKI-272) in combination with capecitabine in patients with solid tumors: A phase 1/2 study (abstract 5108). Cancer Res 69(24 suppl), 2009.
31. Awada A, Dirix L, Beck J, et al: Safety and efficacy of neratinib (HKI-272) in combination with vinorelbine in ErbB2+ metastatic breast cancer (abstract 5095). Cancer Res 69(24 suppl), 2009.
32. Baselga J, Gelmon KA, Verma S, et al: Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 28:1138-1144, 2010.
33. Baselga J, Cortes J, Fumoleau P, et al: Pertuzumab and trastuzumab: Re-responses to 2 biological agents in patients with HER2-positive breast cancer which had previously progressed during therapy with each agent given separately: A new biological and clinical observation (abstract 5114). Cancer Res 69(24 suppl), 2009.
34. Chandarlapaty S, King T, Sakr R, et al: Hyperactivation of the PI3K-AKT pathway commonly underlies resistance to trastuzumab in HER2 amplified breast cancer (abstract 709). Cancer Res 69(24 suppl), 2009.
35. O'Regan R, Andre F, Campone M, et al: RAD001 (everolimus) in combination with weekly paclitaxel and trastuzumab in patients with HER-2-overexpressing metastatic breast cancer with prior resistance to trastuzumab: A multicenter phase I clinical trial (abstract 3119). Cancer Res 69(2 suppl), 2009.
36. Fasolo A, Gianni L, Rorive A, et al: Multicenter phase I clinical trial of daily and weekly RAD001 (everolimus) in combination with vinorelbine and trastuzumab in patients with HER-2-overexpressing metastatic breast cancer with prior resistance to trastuzumab (abstract 406). Cancer Res 69(2 suppl), 2009.
37. Konecny GE, Meng YG, Untch M, et al: Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res 10:1706-1716, 2004.
38. Hurvitz SA, Pegram MD, Lin LS, et al. Final results of a phase II trial evaluating trastuzumab and bevacizumab as first line therapy of HER2-amplified advanced breast cancer (abstract 6094). Cancer Res 69(24 suppl), 2009.
39. Slamon D, Gomez HL, Kabbinavar FF, et al: Randomized study of pazopanib + lapatinib vs. lapatinib alone in patients with HER2- positive advanced or metastatic breast cancer (abstract 1016). J Clin Oncol 26(15S):45s, 2008.
40. Dickler M, Franco S, Stopeck A, et al: Final results from a phase II evaluation of lapatinib (L) and bevacizumab (B) in HER2-overexpressing metastatic breast cancer (MBC) (abstract 3133). Cancer Res 69(2 suppl), 2009.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Address all correspondence to:
Conleth G. Murphy, MD
Breast Cancer Medicine Service
Memorial Sloan-Kettering Cancer Center
300 East 66th St
New York, NY 10065
e-mail: murphyc@mskcc.org