Herceptin and Tykerb going head-to-head
In one of the most ambitious clinical trial efforts to date, a phase III study involving 8,000 participants in 50 countries across six continents will provide a head-to-head comparison of trastuzumab (Herceptin) and lapatinib (Tykerb) in HER2-positive patients with early-stage breast cancer who have recently completed chemotherapy.
NEW YORK-In one of the most ambitious clinical trial efforts to date, a phase III study involving 8,000 participants in 50 countries across six continents will provide a head-to-head comparison of trastuzumab (Herceptin) and lapatinib (Tykerb) in HER2-positive patients with early-stage breast cancer who have recently completed chemotherapy.

Although both agents have FDA approval for use in HER2-positive breast cancer, this will be the first effort to compare the drugs’ efficacy, and also is one of first trials of lapatinib in early-stage disease.
The Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO) study will combine the resources of two large academic breast cancer networks-The Breast Cancer Intergroup of North America (TBCI) based in the United States, which consists of six NCI-funded clinical trials groups, and the Belgium-based Breast International Group (BIG) network.
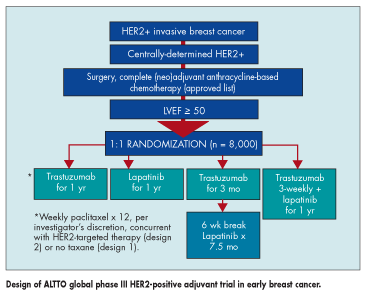
Patients in the trial must have received an approved anthracycline-based adjuvant or neoadjuvant chemotherapy regimen for stage I-II breast cancer.

They will be randomized to one of four arms for a period of 1 year: trastuzumab or lapatinib alone, trastuzumab followed by lapatinib, or the two treatments in combination. The trial has two further designs based on whether patients receive no paclitaxel (design 1) or paclitaxel concurrent with HER2-targeted therapy (design 2), at the investigator’s discretion (see Figure).
A powerful strategy
“Using two treatments that work in different ways against HER2-positive breast cancer may offer a more powerful strategy than either drug alone,” Edith Perez, MD, leader of the TBCI branch of the study, said at a writer’s seminar.
Dr. Perez, of the Mayo Cinic, Jacksonville, Florida, is in the North Central Cancer Treatment Group (NCCTG), which will act as the treatment base for ALTTO in North America.
Since trastuzumab, an IV monoclonal antibody, and lapatinib, an oral small- molecule tyrosine kinase inhibitor, have different side-effect profiles, “it is unlikely that the drugs will be too toxic when taken together,” Dr. Perez said.
In the past, she said, “we used many similar and somewhat repetitive smaller trials to address each question. However, we’ve learned that each clinical issue needs large-scale randomized evidence, preferably gathered from tens of thousands of patients.”
Thousands of biological samples
According to the study’s leaders, ALTTO will be the first clinical trial with such large reach among disparate populations in which the principles of translational research play a prominent role.
To that end, the researchers will collect thousands of biological patient samples to determine a tumor profile that would have the best response with a corresponding drug.
“The difference between this study and most that came before it is that the collection of biological materials occurs as the trial is being conducted, not as an afterthought. Very few organizations have been willing to make the considerable investment in this kind of wide-reaching research,” said Martine J. Piccart, MD, PhD, lead investigator with BIG, which she founded in 1996, and professor of oncology, Universite Libre de Bruxelles, Belgium.
Sponsored by NCI and GlaxoSmithKline, ALTTO is scheduled to end in 2011 with an approximate cost of $100 million.
More than 300 centers around the world have enrolled patients, with full enrollment expected to involve about 500 centers in the United States and more than 800 centers in Europe and other parts of the globe.