High-Dose Chemotherapy Plus Rituximab Produces High Complete Response Rate
HOUSTON-High-dose chemotherapy (HDCT) plus rituximab (Rituxan) produces responses comparable to HDCT with total body irradiation and stem cell transplant for aggressive mantle cell lymphoma (MCL), according to Jorge E. Romaguera, MD, of the University of Texas M. D. Anderson Cancer Center in Houston, Texas. In a poster presentation, Dr. Romaguera said that HDCT with rituximab (but without total body irradiation or stem cell transplant) produced a complete response (CR) rate of 86%.
HOUSTONHigh-dose chemotherapy (HDCT) plus rituximab (Rituxan) produces responses comparable to HDCT with total body irradiation and stem cell transplant for aggressive mantle cell lymphoma (MCL), according to Jorge E. Romaguera, MD, of the University of Texas M. D. Anderson Cancer Center in Houston, Texas. In a poster presentation, Dr. Romaguera said that HDCT with rituximab (but without total body irradiation or stem cell transplant) produced a complete response (CR) rate of 86%.
The failure rate at 9 months median follow-up was comparable to that observed with stem cell transplant (6% vs 4%) and much better than the 50% failure rate seen with historical CHOP (cyclophosphamide, doxorubicin, Oncovin [vincristine], prednisone) controls, he added.
"Patients with mantle cell lymphoma have a poor prognosis. Anthracycline-containing chemotherapeutic regimens typically provide low response rates with only 21% complete response, a short median response duration of 10 months, and dismal survival rates of 2 to 4 years," Dr. Romaguera said. "A recent CHOP-like regimen of hyperfractionated cyclophosphamide, doxorubicin, vincristine, and dexamethasone [HCVAD] alternating with high-dose methotrexate [M] and cytarabine (Ara-C) [A] and consolidated after four courses with high-dose cyclophosphamide, total body irradiation, and autologous blood or marrow stem cell transplantation can achieve 100% CR."
In this earlier study, seven patients did not receive transplant because of financial reasons, inability to harvest stem cells, or refusal. Instead, they received up to eight cycles of HCVAD/M-A, which produced an 86% CR rate with comparable time to progression.
Encouraging Results
"Because of this, we are currently investigating eight alternating cycles of HCVAD/M-A in previously untreated patients but without consolidation unless a CR is not achieved after the first six cycles of treatment," Dr. Romaguera said. "We have included rituximab at 375 mg/m2 given 24 hours before each of the first 6 cycles of therapy." (See Figure 1.) The dose of cytarabine was limited to 1 g/m2/dose in patients over age 60 or those having elevated serum creatinine levels.
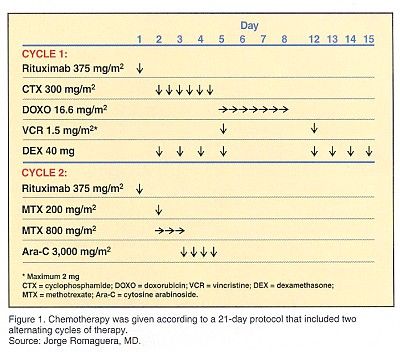
Patients were restaged at the end of the first two cycles of therapy. In the case of CR, patients were given four more cycles of therapy. In the case of partial response (PR), patients were given six more cycles of therapy. If patients were still in PR after six cycles of therapy, they were taken off study and offered stem cell transplantation.
Prophylactic granulocyte-colony stimulating factor (G-CSF), fluconazole (Diflucan), valacyclovir (Valtrex), and levofloxacin (Levaquin) were given on days 8-17 in cycle 1 and days 5-14 of cycle 2.
Data on 49 evaluable patients were reported, including 37 who completed six cycles of therapy. Dr. Romaguera reported a CR rate of 86%, an 11% PR rate, and 3% failures. "HCVAD/M-A with rituximab achieves a high rate of complete remission," he said. "Hematologic toxicity was severe, but only 8% of patients had grade 3 infections according to NCI criteria. These results are encouraging and the trial continues to accrue patients."