Rituximab Plus Fludarabine May Be Good Alternative to Rituximab Plus CHOP
BUFFALO-Combining rituximab (Rituxan) with fludarabine (Fludara) for low-grade or follicular B-cell lymphomas may be as effective as but less toxic than rituximab plus CHOP (cyclophosphamide, doxorubicin, Oncovin [vincristine], prednisone). Phase II trial data supporting this assertion were presented.
BUFFALOCombining rituximab (Rituxan) with fludarabine (Fludara) for low-grade or follicular B-cell lymphomas may be as effective as but less toxic than rituximab plus CHOP (cyclophosphamide, doxorubicin, Oncovin [vincristine], prednisone). Phase II trial data supporting this assertion were presented.
Although initial problems with hematologic toxicity required changes in the planned regimen, including a 40% dose reduction in fludarabine for some patients, lead investigator Myron Czuczman, MD, said that the rituximab/fludarabine combination produced an overall response rate of 93% while preserving immunoglobulin levels and natural killer (NK) cells. Dr. Czuczman is assistant professor of clinical medicine and head of the Lymphoma Section at Roswell Park Cancer Institute, Buffalo.
The rationale for combining these two drugs was that fludarabine has demonstrated single-agent efficacy in chronic lymphocytic leukemia and in refractory or recurrent low-grade B-cell non-Hodgkin’s lymphoma (NHL). Fludarabine inhibits DNA synthesis in sensitive cells. Rituximab has demonstrated single-agent efficacy against B cells. The two drugs have mechanisms of action that are not cross resistant and have no apparent or significant overlapping toxicities. Dr. Czuczman also said that in vitro studies have demonstrated synergistic antitumor activity when fludarabine and rituximab are combined.
Goal and Objectives
The goal of this study was "to maintain excellent antitumor activity with less nonhematologic toxicity than observed in the recent CHOP plus rituximab trial.’’ The main study objectives were to evaluate the safety and efficacy of the combination regimen in treatment-naive, recurrent, or refractory patients with low-grade or follicular NHL and to evaluate changes in lymphocyte subsets (especially NK cells) during treatment and follow-up.
The pilot study has completed enrollment of 40 patients as originally planned, and data on 30 evaluable patients were presented at the meeting.
Inclusion criteria were:
low-grade or follicular NHL;
measurable tumor;
Karnofsky performance status > 60%;
no prior treatment or up to four prior standard chemotherapies; and
adequate hematologic, renal, and hepatic function
Exclusion criteria included:
positive for HIV;
primary or secondary CNS lymphoma; or
prior treatment with fludarabine or any other purine antimetabolite, or with prior anti-CD20 immunotherapy.
The first 10 patients in this open- label, single-arm, single-center phase-II study were treated with the planned regimen of seven doses of rituximab (375 mg/m2/dose) in combination with six cycles of fludarabine (25 mg/m2/d ´ 5 days q 28 days). Two infusions of rituximab were given at the beginning and end of therapy and single infusions of rituximab were given prior to the second, fourth, and sixth cycles of fludarabine. Trimethoprim-sulfamethoxazole (Bactrim) was given as prophylaxis. Growth factor support was not routinely given.
Significant Neutropenia
Hematologic toxicity in the first 10 patients treated caused the investigators to revise the treatment regimen. Two of the first 10 patients discontinued treatment due to cytopenia. Adverse events included grade IV neutropenia in four patients, neutropenic fever with negative cultures in two patients, and need for G-CSF/GM-CSF support in seven patients. Limited herpes zoster infections also developed in 2 of 10 patients.
"Unique to the rituximab plus fludarabine combination was the observation of significant neutropenia in the first 10 patients treated, which led to discontinuation of prophylactic Bactrim, limited use of growth factors, and if needed, a 40% reduction of fludarabine in patients with prolonged cytopenia," Dr. Czuczman said.
Six of the seven patients who completed therapy with the original regimen had complete responses, and one had a partial response. Of the 20 patients treated with the revised regimen, only four required transient growth factor support. Seven patients developed grade IV neutropenia, and one discontinued treatment as a consequence. Only one patient had neutropenic fever with negative culture. One patient discontinued treatment due to cytopenia. Three patients developed herpes zoster. Fludarabine dose reductions were required for three patients.
Excellent Antitumor Activity
Sixteen of the 17 patients who completed treatment with the revised regimen had complete responses, and one patient had a partial response. Considering all 30 treated patients, the overall response rate was 93%. This included 80% complete responses and 13% partial responses. The median duration of response is 14+ months, ranging from 4 to over 26 months, according to Dr. Czuczman.
He said that 89% of patients showed nodal responses in the midtreatment restaging studies and 24 of 28 patients maintain ongoing responses. Polymerase chain reaction studies showed elimination of cells with the bcl-2 (t[14;18]) rearrangement from peripheral blood in 9 of 9 patients studied and from bone marrow in 6 of 7 patients studied, Dr. Czuczman reported.
A striking characteristic of this regimen was its ability to preserve immunoglobulin levels and NK cell numbers. No significant change in quantitative serum immunoglobulins occurred in 17 of 26 patients studied, and immunoglobulin levels actually improved in two of 26 patients. Decreases in levels of IgM, IgG, or IgA were seen in seven patients.
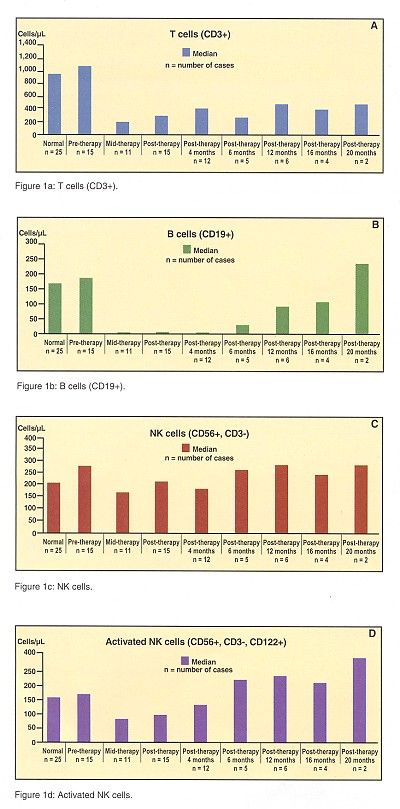
Multiparameter flow cytometry was used to evaluate the effect of rituximab/fludarabine on peripheral blood lymphocyte subsets. According to Dr. Czuczman, "CD3+ T cells were significantly decreased in the majority of patients (Figure 1a). B cells (CD19+ and CD20+) were essentially depleted from peripheral blood in all cases (Figure 1b). NK cells and activated NK cells were essentially spared in the majority of patients (Figures 1c and 1d)."
The most common adverse events attributed to rituximab were fever and chills, observed primarily with the first infusion.
"In summary, interim results of rituximab plus fludarabine combination therapy demonstrate excellent antitumor activity with acceptable toxicity. This is a novel approach for the treatment of indolent NHL," Dr. Czuczman concluded.