High-risk DLBCL yields to dose-dense rituximab regimen
CHICAGO-Increasing the dose density of rituximab in the R-CHOP-14 regimen yields better rituximab (Rituxan) pharmacokinetics and improved clinical outcomes among older adults with high-risk diffuse large B-cell lymphoma (DLBCL), reported lead investigator Michael G.M. Pfreundschuh, MD.
ABSTRACT: The tradeoff of the DENSE-R-CHOP-14 regimen appears to be increased, but manageable, toxicity.

CHICAGO-Increasing the dose density of rituximab in the R-CHOP-14 regimen yields better rituximab (Rituxan) pharmacokinetics and improved clinical outcomes among older adults with high-risk diffuse large B-cell lymphoma (DLBCL), reported lead investigator Michael G.M. Pfreundschuh, MD.
Increasing the density of rituximab in R-CHOP is an attractive approach for improving efficacy in older patients because of this agent’s low toxicity, explained Dr. Pfreundschuh, a hematologist-oncologist at the Saarland University Medical School in Homburg, Germany.
He noted that even with biweekly rituximab administration in the R-CHOP-14 regimen, serum rituximab levels rose slowly. He and his colleagues therefore tested the DENSE-R-CHOP-14 regimen, which delivers four additional rituximab doses in the first month of therapy.
In the trial, 125 patients (ages 61 to 80) with DLBCL of any stage received DENSE-R-CHOP-14, consisting of six cycles of biweekly CHOP and 12 doses of rituximab. Fifty-eight percent of the patients had high-risk disease (IPI of 3, 4, or 5), while the rest had low-risk disease (IPI of 1 or 2)
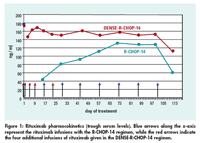
.
Pharmacokinetic results, the study’s primary endpoint, showed more rapid achievement of higher levels of rituximab with the new regimen than with conventional R-CHOP-14 seen historically, Dr. Pfreundschuh reported (see Figure 1).
Surprisingly, an interim analysis of the first 20 patients showed high rates of grade 3/4 infections (7 patients), interstitial pneumonia (7 patients), and death during complete remission (3 patients), with at least some of the pneumonias apparently due to reactivation of cytomegalovirus and Pneumocystis carinii, he noted during a presentation at the 2008 ASCO meeting (abstract 8508). Prophylaxis with acyclovir and cotrimoxazole was mandated for the remaining patients, with a subsequent reduction in grade 3/4 infections, he said.
When compared with patients in the RICOVER-60 trial who received the same number of biweekly CHOP cycles but only eight doses of rituximab (Pfreundschuh M et al: ASH 2006, abstract 205), patients in the DENSE-R trial had a fairly similar rate of complete remission overall (82% vs 78%).
However, stratified analyses showed a selective benefit among high-risk patients (82% vs 68%). “This difference appears to be significant with all the caveats of a historical comparison,” Dr. Pfreundschuh said.
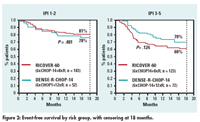
In terms of event-free survival, the DENSE-R regimen was not superior to the RICOVER-60 regimen among low-risk patients (78% vs 81%), but there was a substantial difference among high-risk patients (70% vs 60%) (see Figure 2).
Similarly, DENSE-R conferred no gain in progression-free survival in the low-risk group (87% vs 89%) but a near-significant gain in the high-risk one (80% vs 70%).
“Even though the optimal serum level of rituximab is unknown, our results suggest that at least the high-risk patients benefit from higher rituximab serum levels,” he commented. “We think that these results warrant a randomized comparison.”
Highlighting Insights From the Marginal Zone Lymphoma Workshop
Clinicians outline the significance of the MZL Workshop, where a gathering of international experts in the field discussed updates in the disease state.