HIV Postexposure Prophylaxis Guidelines
ATLANTA-Health care personnel exposed to HIV should be evaluated within hours (rather than days) after their exposure and should be tested for HIV at baseline (ie, to establish infection status at the time of exposure), according to the latest HIV postexposure prophylaxis (PEP) guidelines from the US Public Health Service [MMWR 50:(RR11):1-42, 2001].
ATLANTAHealth care personnel exposed to HIV should be evaluated within hours (rather than days) after their exposure and should be tested for HIV at baseline (ie, to establish infection status at the time of exposure), according to the latest HIV postexposure prophylaxis (PEP) guidelines from the US Public Health Service [MMWR 50:(RR11):1-42, 2001].
Because most occupational HIV exposures do not result in the transmission of HIV, potential toxicity must be carefully considered when prescribing PEP (see Table). Health care personnel with occupational exposure to HIV should receive follow-up counseling, postexposure testing, and medical evaluation, regardless of whether they receive PEP. For management resources, see PEPline (www.ucsf.edu/hivcntr) and Needlestick! (www.needlestick.mednet.ucla.edu).
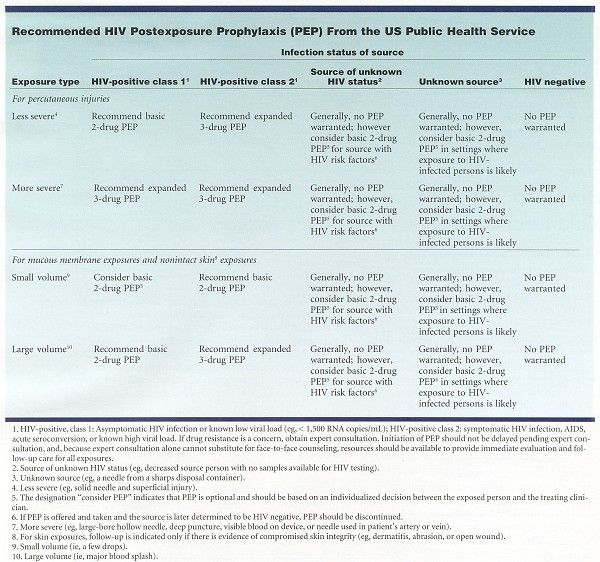
The basic 2-drug PEP regimens, given for 4 weeks, as recommended in the Table, are zidovudine (Retrovir)/lamivudine (Epivir) (available as Combivir); lamivudine/stavudine (Zerit); and didanosine (Videx)/stavudine.
The expanded 3-drug regimen consists of a basic regimen plus indinavir (Crixivan), nelfinavir (Viracept), efavirenz (Sustiva), or abacavir (Ziagen). Abacavir is available in combination with zidovudine and lamivudine as Trizivir.
Drugs for use as PEP with expert consultation include ritonavir (Norvir), saquinavir (Fortovase), amprenavir (Agenerase), delavirdine (Rescriptor), and lopinavir/ritonavir (Kaletra).