Hypothyroidism:A Growing Complicationof Cancer Treatment
Hypothyroidism is a common and potentially serious endocrine disorder in the general population.
Hypothyroidism is a common and potentially serious endocrine disorder in the general population. It is more common in women, the elderly, and whites. In the United States, the incidence in women over the age of 60 years is up to 20%, and in men over the age of 74 years it is up to 15%.[1]
Common causes of hypothyroidism include inadequate levels of dietary iodine intake, pregnancy, radiotherapy to the thyroid gland, viral infections, surgery, infiltrative disorders including lymphoma, carcinoma and iron overload, autoimmune diseases, type I diabetes, administration of amiodarone or lithium, and rare congenital factors.[2]
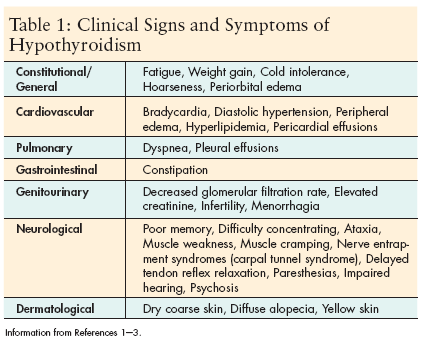
The physiological effects of hypothyroidism are widespread and include cardiovascular, renal, gastrointestinal, neurological, and endocrine abnormalities. The clinical manifestations range from asymptomatic individuals to severe symptoms including congestive heart failure, adrenal insufficiency, psychosis, and coma (Table 1).[1]
Thyroid dysfunction as a result of cancer treatment is an often overlooked clinical problem. While the incidence with use of standard chemotherapeutic agents is uncommon, thyroid dysfunction from neck irradiation, immune therapy (interleukin-2 [aldesleukin; Proleukin], interferon), immunomodulatory agents (thalidomide [Thalomid], lenalidomide [Revlimid]), radioimmunoconjugates (131I-tositumomab [Bexxar]) and small-molecule inhibitors (sunitinib [Sutent], sorafenib [Nexavar]) may result in either hyperthyroidism or hypothyroidism (Table 2).
The signs and symptoms of hypothyroidism may be attributed to treatment with these agents, and unnecessary dose modifications or discontinuation of potentially beneficial therapy may result. A review of hypothyroidism, including presenting signs and symptoms, indications for treatment, and implications for the patient with cancer will assist in effective management of this very treatable disorder.
NORMAL THYROID FUNCTION
The function of the thyroid gland is to take iodine, found in many foods, and convert it into thyrotropin-releasing hormones thyroxine (T4) and triiodothyronine (T3). A euthyroid state (normal thyroid function) is maintained by a feedback loop between the anterior pituitary gland, which produces thyroid-stimulating hormone (thyrotropin; TSH) in response to thyrotropin-releasing hormones produced by the thyroid gland.[2] All cells in the body depend upon thyroid hormones for regulation of their metabolism.
The normal thyroid gland produces about 80% T4 and about 20% T3.[3] The majority of T4 is bound to proteins, with approximately 0.3% free in the circulation (free T4). Free T4 is metabolically active. T3 is four times more potent than T4, but it is secreted in very small quantities by the thyroid. Elevated levels of T4 and T3 inhibit further release of TSH from the pituitary, completing the feedback loop.[2]
The diagnosis of hypothyroidism is confirmed by measurement of serum TSH and free thyroxine (T4). Normal serum thyrotropin levels range from 0.4–4.5 mU/L. Administration of glucocorticoids, dopamine, and dobutamine may interfere with accurate testing.[1] Primary hypothyroidism is characterized by a low free T4 level and elevated TSH. Central hypothyroidism is associated with low free T4 and inappropriately normal or low TSH levels. Primary hypothyroidism may be overt (clinical) or mild (subclinical). Replacement with levothyroxine is indicated for patients who have symptomatic hypothyroidism with a serum TSH > 10 µIU/mL or in patients with goiter and positive antithyroid peroxidase antibodies. Treatment is generally highly effective. Subclinical hypothyroidism, characterized by an elevated TSH and normal T4 level, is common but may
not require immediate therapy in asymptomatic individuals.[1–3]
HYPOTHYROIDISM CAUSED BY CANCER TREATMENT Radiation
Irradiation of the thyroid gland may produce hypothyroidism, Graves' disease, silent thyroiditis, benign nodules, and thyroid cancers. Radiation-induced thyroid dysfunction is thought to be caused by damage to the small thyroid vessels and to the glandular capsule.[4] The total dose of irradiation has been shown to correlate with the incidence of hypothyroidism. In patients with Hodgkin's disease, the risk of hypothyroidism at 20 years from the time of diagnosis in those treated with 45 Gy or more was 50%.[5] Patients receiving mantle irradiation or radiation to the cervical region need careful monitoring for thyroid abnormalities. Concurrent administration of chemotherapy may increase the risk substantially.
Administration of the agent 131I-tositumomab, a radioimmunoconjugate used to treat non-Hodgkin's lymphoma, is known to have a direct effect on the thyroid tissues.[6,7] The administration of thyroid-protective agents at least 24 hours prior to administration of the radioactive iodine 131I is necessary to prevent thyroid ablation.
The available thyroid-protective agents are saturated solution of potassium iodide (SSKI) given at a dosage of four drops orally three times daily; Lugol's solution at 20 drops orally three times daily; or potassium iodide tablets at a 130-mg dose orally daily. Thyroid- protective agents should be initiated at least 24 hours prior to administration of the 131I-tositumomab dosimetric dose and continued until 2 weeks after administration of the therapeutic dose.[6]
Chemotherapy
Clinical hypothyroidism is rarely associated with standard doses of chemotherapy. However, with the high doses used for conditioning regimens administered prior to bone marrow transplantation, the incidence can approach 50%.[8] Transient alterations in thyroid hormone blood levels have been noted with use of 5-fluorouracil and with L-asparaginase (Elspar). These do not generally require treatment.[9] In postmenopausal women, tamoxifen therapy is associated with changes in thyroid hormone concentrations, although patients may remain clinically euthyroid.[10] Autoimmune thyroiditis is most common in elderly women, placing elderly females on tamoxifen at particular risk.[1]
Biological agents/targeted therapies
Hypothyroidism associated with administration of interleukin-2 is thought to be caused by autoreactive B-cell clones and cytokine-mediated thyroid destruction resulting in development of antithyroid antibodies and secondary thyroiditis.[9] Thalidomide and lenalidomide, both immunomodulatory agents, have been associated with hypothyroidism when used in the treatment of various hematological malignancies or solid tumors, suggesting a class effect.[9] Although the mechanism is not fully understood, it is thought to be a result of immune-mediated thyroiditis. Hypothyroidism is a frequent complication of sunitinib treatment used for either renal cell carcinoma or gastrointestinal stromal tumors (GIST), with incidence rates ranging from 17%–81%.[9,11,12]
Inhibition of vascular endothelial growth factor (VEGF), augmentation of T-cell immune function, drug-induced thyroid atrophy, and inhibition of iodine uptake in the thyroid have been suggested as possible factors contributing to hypothyroidism associated with biological and targeted agents.[1,7,9,11,12] Bexarotene (Targretin), a retinoid derivative used in treatment of T-cell malignancies, can cause thyroid axis alterations and a reversible reduction in thyroid hormone levels. Many of these patients will require therapy with levothyroxine.[13]
Treatment
Treatment of hypothyroidism is based on replacing thyroxine. Treatment indications recommended by the American Association of Clinical Endocrinologists have been previously discussed.[3] The National Cancer Institute Common Toxicity Criteria (NCI-CTC) for grade 3–4 hypothyroidism are vague and do not indicate specific laboratory parameters. Grade 3 hypothyroidism is defined as symptomatic, not interfering with activities of daily living (ADL) but requiring thyroid replacement. Grade 4 hypothyroidism is defined as symptomatic, interfering with ADL, and/or requiring hospitalization.
Given the variability in available approaches to treatment, these parameters require that symptoms be attributed to the thyroid dysfunction as opposed to the active treatment or other causes. Therefore, the recommendations are to obtain baseline TSH levels on all patients who are elderly or who will be receiving treatment with agents/modalities known to be associated with thyroid dysfunction.[3]
If the TSH is abnormal, then assessment of free T4 and T3 levels will be necessary to define the thyroid dysfunction. Normal ranges for T4 are 10–27 pmol/L, and for T3 are 3–9 pmol/L. Levels should be monitored throughout the treatment course, with intervals of 4–6 weeks recommended for agents with high probability of causing thyroid function effects (sunitinib, bexarotene). Less frequent monitoring (every 6 months) is recommended for agents or modalities expected to have latent effects (radiation, bone marrow transplant, 131I-tositumomab). In more severe cases or in cases of suspected autoimmune thyroiditis, assessment of thyroid peroxidase (TPO) levels may be beneficial.
Patients requiring active treatment of hypothyroidism are generally started on levothyroxine at 1.6 µg/kg/day, although doses may vary based on age, weight, cardiac status, and the severity of symptoms.[3] In the elderly or in patients with underlying coronary artery disease, the starting dose is 12.5–25 µg per day owing to the positive inotropic and chronotropic effects of thyroid hormone.[1]
Reassessment of thyroid function should be conducted 6 weeks after initiating treatment of hypothyroidism and 6 weeks after any change in dose. The monitoring intervals can be reduced when a euthyroid state is achieved. Treatment with thyroid hormone replacement should be continued with increased frequency of monitoring and gradual titration over a 60-day period in patients who discontinue active agents. Patients treated with agents/modalities that are associated with latent hypothyroidism will need ongoing monitoring and may require long-term treatment to address their hypothyroidism.
Summary
Hypothyroidism is an often underreported complication of cancer treatment. With the growing number of biological and targeted therapies, hypothyroidism may become a more prevalent clinical problem. Signs and symptoms of hypothyroidism may be easily attributed to the cancer or cancer treatment, leading to unnecessary dose modifications or discontinuation of effective therapies. Furthermore, these symptoms have a negative effect on quality of life. Measurement of TSH levels in at-risk patients prior to initiating treatment and at regular intervals during treatment will allow early intervention and effective treatment of hypothyroidism.
References:
References
1. Roberts C, Ladenson P: Hypothyroidism. Lancet 363(9411):793â803, 2004.
2. Fauci A, Kasper D, Longo D, et al. (eds): Harrison's Manual of Medicine, 17th ed. New York, NY, McGraw Hill, 2008.
3. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract 8(6):457â469, 2002. Available at: http://www.aace.com/pub/pdf/guidelines/hypo_hyper.pdf. Accessed, July 12, 2009.
4. Hancock SL, Cox RS, McDougall IR: Thyroid disease after treatment of Hodgkin's disease. N Engl J Med 325(9):599â605, 1991.
5. Sklar C, Whitton J, Mertens A, et al: Abnormalities of the thyroid in survivors of Hodgkin's disease: Data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab 85(9):3227â3232, 2000.
6. Bexxar prescribing information. Research Triangle Park, NC, GlaxoSmithKline, October 2005. Available at: http://us.gsk.com/products/assets/us_bexxar.pdf. Accessed on July 12, 2009.
7. Gerber D: Targeted therapies: A new generation of cancer treatment. Am Fam Physician 77(3):311â319, 2008.
8. Tauchmanova L, Selleri C, Rosa GD, et al: High prevalence of endocrine dysfunction in long-term survivors after allogeneic bone marrow transplantation for hematological diseases. Cancer 95(5):1076â1084, 2002
9. Torino F, Corsello SM, Longo R, et al: Hypothyroidism related to tyrosine kinase inhibitors: An emerging toxic effect of targeted therapy. Nat Rev Clin Oncol 6(4):219â228, 2009.
10. Kostoglou-Athanassiou I, Ntalles K, Markopoulous C, et al: Thyroid function in post-menopausal women with breast cancer on tamoxifen. Eur J Gynaecol Oncol 19(2):150â154, 1998.
11. Desai J, Yassa L, Marqusee E, et al: Hypothyroidism after treatment for patients with gastrointestinal stromal tumors. Ann Intern Med 145(9):660â664, 2006.
12. Rini BI, Tamaskar I, Shaheen P, et al: Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Nat Cancer Inst 99(1):81â83, 2007.
13. Targretin (bexarotene) capsules prescribing information. Woodcliff Lake, NJ, Eisai Inc., January 2007.
14. Hutson TE, Figlin RA, Kuhn JG, et al: Targeted therapies for metastatic renal cell carcinoma: An overview of toxicity and dosing strategies. The Oncologist 13(10):1084-1096, 2008.