Idelalisib/Rituximab Combo Improves Survival in Late-Stage CLL
A phase III trial of idelalisib plus rituximab in patients with previously treated chronic lymphocytic leukemia found that the combo improved progression-free and overall survival, according to results presented at the 2013 ASH meeting.
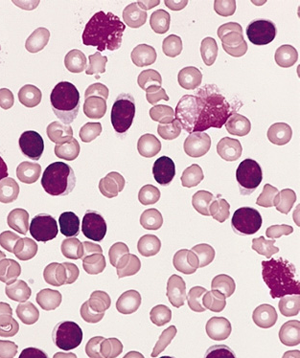
Blood smear from an adult male with CLL, Wright-Giemsa stain.
Idelalisib plus rituximab may soon be a new option to treat patients with previously treated chronic lymphocytic leukemia (CLL), who are not eligible for chemotherapy.
The progression-free survival (PFS) rate at 24 weeks was 93% for patients treated with idelalisib plus rituximab compared with 46% for patients treated with rituximab alone. The median PFS of the combination arm has not yet been reached. The median PFS in the rituximab alone arm is 5.5 months (hazard ratio [HR] = 0.15; P < .0001).
Patients in the idelalisib arm also had an improved overall survival compared with patients in the rituximab-alone arm (HR = 0.28; P = .018).
Results from Study 116, a randomized phase III trial, were presented at the late-breaking abstracts session of the 2013 American Society of Hematology (ASH) Annual Meeting and Exposition, held in New Orleans.
Idelalisib is a novel, first-in-class oral kinase inhibitor that specifically inhibits the delta isoform of the PI3 kinase found predominantly in leukocytes but not other cell types.
In October, this trial was stopped early based on a recommendation by an independent Data Monitoring Committee following an interim analysis that showed the combination of idelalisib plus rituximab resulted in a statistically significant increase in PFS compared with rituximab alone.
“This combination looks extremely good in terms of response rates and adverse events,” said Jennifer R. Brown, MD, PhD, a CLL and lymphoma specialist at the Dana-Farber Cancer Institute and assistant professor of medicine at Harvard Medical School in Boston, who was not involved in the study.
The trial enrolled 220 recurrent patients who had measurable lymphadenopathy and were previously treated but had progressed less than 24 months after completing treatment. Patients were not fit enough to receive cytotoxic therapy to treat their CLL due to comorbidities, such as cytopenia and renal dysfunction.
Patients were randomized 1:1 to receive either rituximab alone at 375 mg/m2 in the first dose and 500 mg/m2 for subsequent doses for a total of 8 doses plus a placebo, or rituximab plus idelalisib at a dose of 150 mg twice daily.
The median age of patients was 71 years, and they had received a median of three prior therapies. Forty-four percent of the patients had either a 17p deletion or a p53 mutation, and 84% had a nonmutated immunoglobulin heavy chain variable region (IgVH) gene.
In a prespecified high-risk subgroup analysis, PFS favored the idelalisib plus rituximab arm for poor prognosis patients with either a 17p deletion or p53 mutation (HR = 0.12).
The overall response for patients treated with the combination was 81% compared with 13% in the control arm (odds ratio [OR] = 29.92; P < .0001). Patients treated with idelalisib plus rituximab also had a higher lymph node response (93%) compared with 4% in the rituximab arm (OR = 264.5; P < .0001).
“All patients in the idelalisib arm had some decrease in lymphadenopathy, with 93% having at least a 50% decrease, which is a nodal response,” said Richard R. Furman, MD, of Weill Cornell Medical College in New York City, who presented the data at a press briefing at the ASH meeting.
Adverse events were similar in both treatment arms. Serious adverse events occurred in 40% of patients in the combination arm and 34.6% in the control arm. Adverse events that led to trial discontinuation occurred in 8.2% and 10.3% in the experimental and control study arms.
“Common adverse events were similar in both treatment groups with one interesting exception-infusion-related reactions were much higher in the placebo plus rituximab arm,” said Dr. Furman during the press briefing. Common adverse events included pyrexia, fatigue, nausea, and chills.
“It is still too early to know what the full durability will be, but the response rate is very high thus far. We will need longer follow-up, which is ongoing, to understand the durability,” Dr. Brown told Cancer Network.
A New Drug Application for approval of idelalisib for treatment of indolent non-Hodgkin lymphoma refractory to rituximab was filed in September with the US Food and Drug Administration. Idelalisib is also being tested in several phase III trials in combination with other agents for CLL.