Imatinib readies patients with Philadelphia-positive ALL for allogeneic stem cell transplant
Patients in a major UK/US trial who received imatinib had improved overall survival, event-free survival, and relapse-free survival at three years of follow-up compared with patients who did not receive any imatinib.
ORLANDO, FLA-Final results of a joint study by British and American researchers demonstrated that treatment with imatinib (Gleevec) enhanced survival in patients with Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) who undergo allogeneic stem cell transplant.
ALL is the most common type of leukemia, with almost 4,000 new cases diagnosed each year in the U.S. Up to 25% of all adults with ALL will suffer from Ph+ ALL, a particularly aggressive form of the disease that does not respond to induction chemotherapy in terms of achieving prolonged remission.
As a result, allogeneic stem cell transplant is often recommended after the first complete remission, said Adele K. Fielding, MBBS, PhD, lead investigator of the UKALLXII/ECOG2993* trial. The trial was started in 1993 to explore whether allogeneic stem cell transplant would be an effective treatment option for adult patients with Ph+ ALL.
In this part of the study, which began before imatinib became the standard of care, 266 patients with Ph+ ALL received two phases of induction chemotherapy followed by an allogeneic stem-cell transplant whenever possible. Once imatinib became available, the study was changed to evaluate the drug’s use as part of consolidation therapy prior to an allogeneic stem-cell transplant or as part of standard induction therapy before the transplant.
Patients in the trial were divided into three categories:
• Pre-imatinib patients were those who received stem cell transplant with no imatinib
• Late-imatinib patients were those who received imatinib as part of consolidation therapy prior to transplant
• Early-imatinib patients were those who received imatinib as part of standard induction therapy before the transplant
In the late-imatinib arm, 86 patients received imatinib 600 mg a day as part of consolidation therapy prior to undergoing an allogeneic stem cell transplant following two induction chemotherapy regimens. The 89 patients in the early-imatinib arm were given imatinib 600 mg earlier in the treatment cycle, as part of the second chemotherapy induction phase prior to the allogeneic stem cell transplant. In addition, all patients who received a transplant during the study were given imatinib for two years post-transplant. If transplant was not feasible, patients were given imatinib as maintenance therapy for two years.
In the pre-imatinib arm of the trial, only 28% of patients were able to go on to receive allogeneic stem cell transplant. But with any imatinib treatment, 44% of patients successfully received allogeneic stem cell transplant.
Dr. Fielding reported that patients who received imatinib had improved overall survival (OS), event-free survival (EFS), and relapse-free survival (RFS) at three years of follow-up compared with patients who did not receive any imatinib (see table, below).
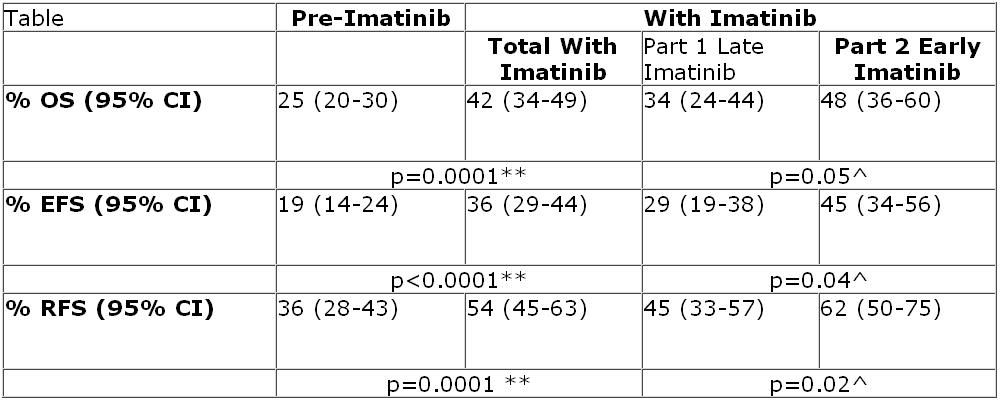
“Although there have been a number of papers published detailing the value of imatinib, this is the largest study to date to compare outcomes with and without imatinib in Ph+ ALL patients. This allows us to make some sensible conclusions,” said Dr. Fielding, a senior lecturer at University College London. Namely, that imatinib increases the rate of complete remission without increasing induction mortality.
“It is this that enables patients to have a high rate of allogeneic bone marrow transplantation. And in this study, compared with large historical controls on the same protocol, there was a definite and significantly improved outcome in all the endpoints measured at three years,” she said.
However, “whether the superior outcome with imatinib was due to a beneficial effect of imatinib per se or the fact that it allows a greater number of patients to go on to receive allogeneic bone marrow transplantation is not yet quite clear,” she added.
Dr. Fielding told Oncology NEWS International that when the study included patients who were not able to proceed to allogeneic bone marrow transplantation, there was only a very modest benefit to having early imatinib. “It is likely that the long-term benefits of imatinib probably relate to a higher rate of allogeneic bone marrow transplantation,” she said.
*UKALLXII/ECOG2993 was a joint effort of the UK’s National Cancer Research Institute and the U.S. Eastern Cooperative Oncology Group (ECOG). Related Reading
Imatinib-based regimen allows stem cell transplant in majority of Philadelphia-positive ALL patients