Immunotoxin Induces Remission in Most Refractory Hairy Cell Leukemia Patients
BETHESDA-"BL22 is the first agent since purine analogs capable of inducing complete remission in the majority of patients with hairy cell leukemia, and the only agent that can induce complete remission in most patients with chemotherapy-refractory or variant HCL," according to Robert J. Kreitman, MD. "Its sparing of T cells," he continued, "may also allow improved clearing of minimal residual disease."
BETHESDA"BL22 is the first agent since purine analogs capable of inducing complete remission in the majority of patients with hairy cell leukemia, and the only agent that can induce complete remission in most patients with chemotherapy-refractory
or variant HCL," according to Robert J. Kreitman, MD. "Its sparing of T cells," he continued, "may also allow improved clearing of minimal residual disease."
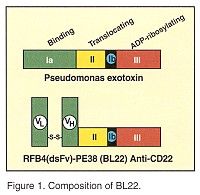
Dr. Kreitman, who is chief of the Clinical Immunotherapy Section at the National Cancer Institute’s Laboratory of Molecular Biology in Bethesda, described results of a phase-I trial of BL22 (RFB4(dsFv)-PE38), a recombinant disulfide-stabilized immunotoxin composed of the variable domains of the anti-CD22 monoclonal antibody RFB4 attached together by a disulfide bond and fused to truncated Pseudomonas exotoxin. (See Figure 1.) Dr. Kreitman said that the investigators’ goal was to "use the extreme potency of Pseudomonas exotoxin, which can kill cells with only one molecule in the cytoplasm."
Low Immunogenicity
This phase-I study enrolled 31 patients with chemotherapy-refractory hairy cell leukemia (HCL, n = 16), chronic lymphocytic leukemia (CLL, n = 11), or non-Hodgkin’s lymphoma (NHL, n = 4) who had CD22 expression on their malignant cells. Each patient received BL22 at 3-50 µg/kg IV every other day for three doses. Thus far, the investigators have administered up to 12 cycles of treatment per patient (range, 1-12).
"Only 3 of 31 patients made neutralizing antibodies, which indicates that this treatment has low immunogenicity and can be given for many cycles," Dr. Kreitman said.
The most common toxicities were hypoalbuminemia, third-spacing of fluid without pulmonary edema, nausea, transaminase elevations, and myalgias. Toxicity was often decreased during re-treatment using anti-inflammatory agents and hydration. The cytokine release syndrome also seen with many monoclonal antibody therapies occurred in only one hairy cell leukemia patient, who had neutralizing antibodies prior to therapy. Dr. Kreitman said that low immunogenicity might be due to the structure of BL22, a small molecule that does not include the IgG constant domains. "Alternately, it might be that these patients are more immunosuppressed than those with solid tumors and less likely to mount an immune response, or that BL22 might have some degree of anti-B-cell activity."
Hemolytic Uremic Syndrome
The dose limiting level was 50 µg/kg qod × 3. One patient developed acute, reversible, renal insufficiency that is now suspected of being hemolytic uremic syndrome (HUS) and two other patients developed reversible HUS. Dr. Kreitman said that this problem was not seen in subsequent patients who had good hydration and no intravenous contrast before BL22. The maximum tolerated dose was 40 µg/kg qod × 3, where all cycles were well tolerated.
"HUS remains a serious potential toxicity," Dr. Kreitman said, "but it may be preventable."
Dr. Kreitman reported that BL22 produced responses in 13 of 16 patients11 complete remissions (CR) and 2 partial responses. All were refractory to purine analog treatment. The 3 nonresponders had either low doses or preexisting neutralizing antibodies. "Most patients had over 90% eradication of hairy cells (demonstrated by flow cytometry) within 2 days and nearly 100% eradication within 1 week of beginning treatment," he said. "Greater disease burden did not prevent the achievement of CR but sometimes required additional cycles of treatment."
All three patients with variant HCL (HCLv) had never been in CR with previous chemotherapy but had CR in response to BL22. Complete responses were most rapid in patients with mono- or oligoclonal elevations in cytotoxic T cells, which often increased with repeated cycles. Pancytopenia reversed in responders and transfusion dependence resolved.
"At a median follow-up of 7 months, there were no relapses from CR," Dr. Kreitman said. "No other agent comes close to the activity of BL22 in this poor-prognosis group, and it is the only agent so far to produce a high CR rate in purine-analog-resistant patients."