Implantable Pump Improves Pain Control, Reduces Toxicity
SAN FRANCISCO-A trial to compare comprehensive medical management (CMM) to CMM plus intrathecal morphine delivery via an implantable, programmable drug delivery system (IDDS) showed that the pump improved pain control by more than 15% and reduced medication side effects by nearly 50%. "The pump not only improved pain control and quality of life in patients with otherwise intractable cancer-related pain," Thomas J. Smith, MD, one of the study chairmen, told ONI, "but reduced costs associated with medication and side effects so much that by the third month of treatment, intrathecal morphine delivery should become cost-effective compared to the high doses of oral morphine typically used in these patients."
SAN FRANCISCOA trial to compare comprehensive medical management (CMM) to CMM plus intrathecal morphine delivery via an implantable, programmable drug delivery system (IDDS) showed that the pump improved pain control by more than 15% and reduced medication side effects by nearly 50%. "The pump not only improved pain control and quality of life in patients with otherwise intractable cancer-related pain," Thomas J. Smith, MD, one of the study chairmen, told ONI, "but reduced costs associated with medication and side effects so much that by the third month of treatment, intrathecal morphine delivery should become cost-effective compared to the high doses of oral morphine typically used in these patients."
Dr. Smith noted that the data were still preliminary. He is chairman of the Division of Hematology/Oncology, Medical College of Virginia Hospitals, Virginia Commonwealth University, Richmond, VA. The study was sponsored by Medtronic.
After Crossover
More than 150 of a planned 200 patients have been enrolled in this first randomized, prospective trial comparing CMM to CMM plus intrathecal morphine administration via the Medtronic SynchroMed pump. Dr. Smith presented preliminary data from 22 patients originally randomized to the CMM arm who subsequently crossed over to the IDDS arm due to intractable pain.
"Since these patients represent the hardest of pain control challenges, we analyzed their response to see if IDDS could relieve their pain," Dr. Smith explained. "All failed pain control or suffered excess side effects on CMM. All centers followed the Agency for Health Care Policy and Research Cancer Pain Relief Guidelines, and all crossovers were centrally approved."
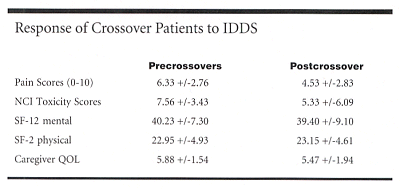
Pain was measured using a visual analog scale (VAS) of 0 to 10. Opioid toxicity was measured using the National Cancer Institute Common Toxicity Criteria.
The average VAS pain score at crossover (6.33 ± 2.76) decreased more than 28% by 1 month after crossover. Toxicity decreased more than 29%. Overall quality of life and caregiver quality of life were similar before and after crossover:
Dr. Smith said that survival after crossover to IDDS was greater than or equal to 3 months, within the range where IDDS can be cost saving compared to conventional medical management in most of the 22 patients.
Some Still Hurting
Study data also show that pain management still leaves some patients suffering despite massive doses of morphine, in both US and European medical centers. The trial also provides a "snapshot" of current pain management for the 10% to 15% of patients not controlled with standard treatment, Dr. Smith noted, adding that the picture is not pretty.
Study physicians predicted overall survival of 10 months, but actual survival was only 3 to 4 months. Neuropathic pain was more common than expected: 59% of patients had mixed neuropathic and nociceptive pain, and an additional 15% had primarily neuropathic pain. Despite use of a mean 880 mg/day of morphine or equivalent, the baseline VAS pain scores were over 7 (on a 0 to 10 scale).
Patients whose pain was not adequately relieved by opioids were using an average of more than 1,200 mg/day at study entry. Baseline quality of life and caregiver quality of life scores were low on both arms, with an average of 3 major toxicities. Most patients were using adjuvant medicines, more so in European (68%) than US (53%) centers.
Substantial Improvements
Patients in both study arms had substantial improvements in pain control while in the study, according to Dr. Smith. "Treatment with intrathecal morphine also sustained these patients’ quality of life at a point in disease progression when quality of life usually declines," Dr. Smith said.
Preliminary data suggest that there is a substantial burden of unrelieved pain in cancer patients despite massive doses of opioids and multiple adjuvant medicines and that morphine administered via an implantable pump can improve pain control for many of these patients. "Early results from this research should encourage more oncologists to consider using implantable programmable drug pumps for intrathecal infusion of morphine to treat patients who continue to experience intractable pain even after traditional pain management," Dr. Smith said. Final study results are expected early in 2002.