Implications of Mutation Profiling in Myeloid Malignancies-PART 2: Myeloproliferative Neoplasms and Other Myeloid Malignancies
In this second part of our two-part review, we discuss the use of mutation profiling in the diagnosis, prognosis, and treatment of patients with myeloproliferative neoplasms and other myeloid diseases.
Oncology (Williston Park). 32(5):e45-e51.

Table 1. Prevalence of Genes Mutated by Disease Type and Stratifi ed by Cellular Function
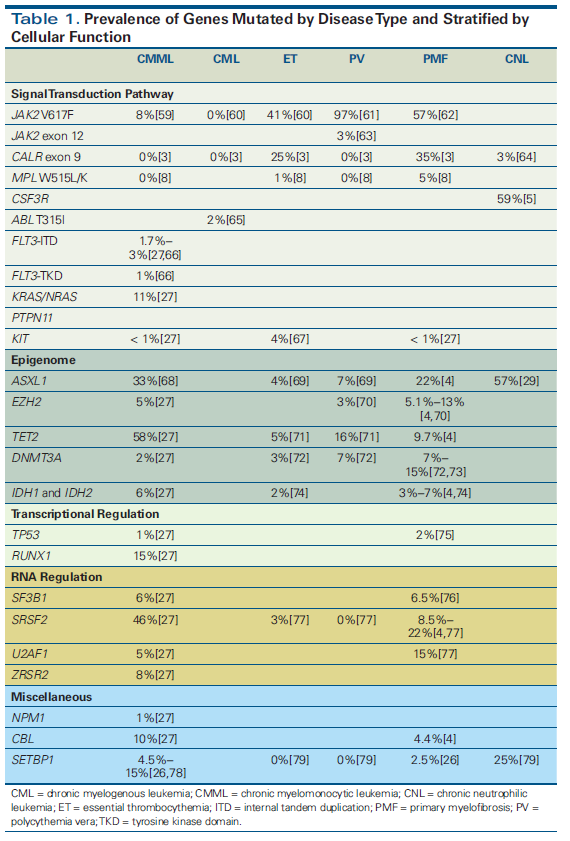
Figure 1. Integrating Genomics Into the Diagnostic Algorithm for the Evaluation of Erythrocytosis
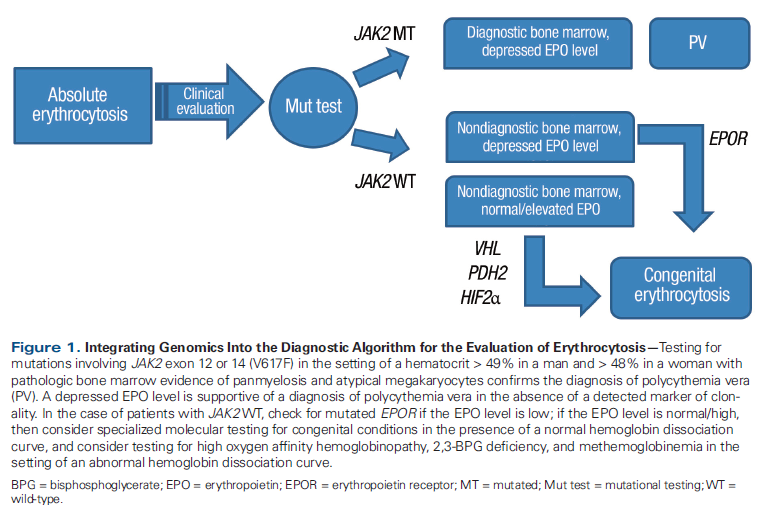
Figure 2. Integrating Genomics Into the Diagnostic Algorithm for the Evaluation of Thrombocytosis
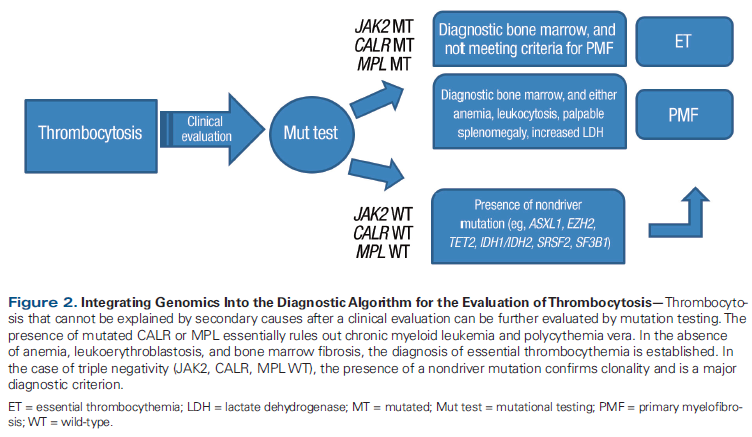
Table 2. Prognostic Implication of Mutations in Patients with Myeloid Malignancies
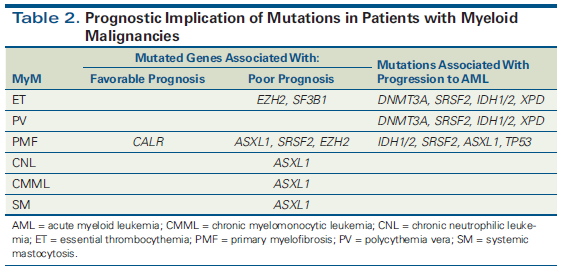
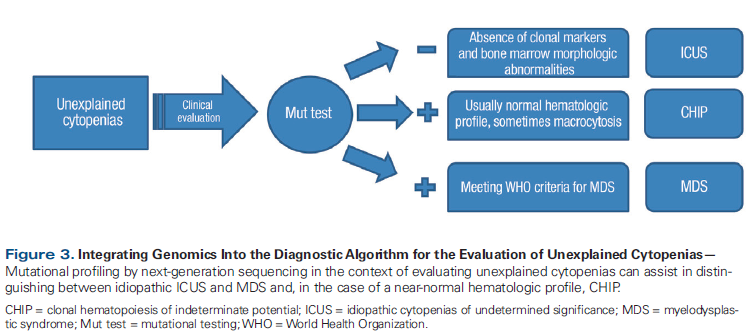
Figure 3. Integrating Genomics Into the Diagnostic Algorithm for the Evaluation of Unexplained Cytopenias
Myeloid malignancies arise from the acquisition of somatic mutations among various genes implicated in essential functioning of hematopoietic stem cells and progenitor cells. In this second part of our two-part review, we discuss the use of mutation profiling in the diagnosis, prognosis, and treatment of patients with myeloproliferative neoplasms and other myeloid diseases. We also discuss the entity known as clonal hematopoiesis of indeterminate potential, awareness of which is a result of the increasing availability and improved quality of mutation profiling.
Myeloproliferative Neoplasms: Diagnostic Implications
Mutation analysis can yield valuable diagnostic information in many patients. As demonstrated in Table 1, mutations are highly recurrent across myeloid malignancies. It is important to emphasize that diagnosis should still rely on established clinicopathologic criteria set forth by the World Health Organization (WHO).[1] As an example, for patients with a suspected myeloproliferative neoplasm (MPN), the presence of mutated CALR and/or absence of mutated JAK2 virtually excludes polycythemia vera as a potential diagnosis, with only rare exceptions.[2,3] Additionally, when determining the subtype of MPN, mutations involving ASXL1 and SRSF2 occur more frequently in primary myelofibrosis than polycythemia vera or essential thrombocythemia.[4] In conjunction with a supportive clinical picture and characteristic bone marrow histomorphology, or the absence of a driver mutation (mutated JAK2, CALR, MPL), these mutations can aid in confirming the diagnosis of primary myelofibrosis. Mutations in CSF3R are frequently found in chronic neutrophilic leukemia,[5,6] and they are now incorporated into the latest WHO criteria for diagnosis.[1] Suggested diagnostic workflows for absolute erythrocytosis and thrombocytosis are presented in Figure 1 and Figure 2.
Myeloproliferative Neoplasms: Prognostic Implications
The classic MPNs include the Philadelphia chromosome (BCR-ABL1)–negative MPNs (polycythemia vera, essential thrombocythemia, primary myelofibrosis) and BCR-ABL1–positive chronic myeloid leukemia (CML). The BCR-ABL1–negative MPNs are genetically characterized by the overlapping presence of mutations in three driver genes-JAK2, CALR, and MPL (which encodes for the thrombopoietin receptor)-in the vast majority of patients (> 90%). Regardless of the driver mutation, resultant hyperactivity of JAK-STAT signaling is observed in these patients and is the central pathogenic theme of BCR-ABL1–negative MPNs. Over 50 different mutations involving CALR have been identified; the most common are type 1, a 52–base pair deletion, and type 2, a 5–base pair insertion.[7] MPL W515L/K is present in MPNs, with a frequency of 4% to 5%, and it can coexist with JAK2 V617F.[8] The presence of these MPN driver mutations is now incorporated into the revised 2016 WHO diagnostic criteria for essential thrombocythemia, polycythemia vera, and primary myelofibrosis.[9]
Knowledge of the driver mutation status and of other key genes in MPN patients provides important prognostic information (Table 2). As mentioned, nearly all patients with polycythemia vera harbor mutations in exon 14 (V617F) and exon 12 of JAK2.[10] In 338 patients with polycythemia vera, a high mutant allele burden (ie, > 50% mutant JAK2) was associated with hemoglobin concentration, leukocyte count, age-adjusted bone marrow cellularity, and spleen size. Importantly, allele burden was significantly associated with progression to myelofibrosis, but not with evolution to acute myeloid leukemia or thrombosis risk.[11] A high allele burden was observed in patients with post–polycythemia vera myelofibrosis, but this in itself did not correlate with worse overall survival (OS).[12]
In essential thrombocythemia, mutation profiling can aid in predicting disease-related complication rates. In an international retrospective study of 891 patients with essential thrombocythemia who were followed up for a median of 6.2 years, the presence of JAK2 V617F was associated with increased risk of developing arterial (not venous) thrombosis (hazard ratio [HR], 2.6; P = .009).[13] In another study of 576 essential thrombocythemia patients, 15.5% harbored a CALR mutation, which conferred a longer thrombosis-free survival when compared with JAK2 V617F– and MPL-mutated patients. There was no association of CALR mutation with OS or transformation to myelofibrosis.[14] More recent data from 502 patients with essential thrombocythemia also demonstrate similar survival in JAK2-, MPL-, and CALR-mutated patients.[15] Another study that applied next-generation sequencing (NGS) to 181 essential thrombocythemia patients demonstrated that the number of mutations present was associated with worse OS (HR, 6.6 for 3 mutations). Additionally, multivariate analysis demonstrated that EZH2 and SF3B1 mutations negatively affected OS.[16] Other inherited constitutional genetic variations may also hold useful prognostic information. For example, the presence of DNA repair gene XPD codon 751 Gln/Gln genotype may predispose to the development of leukemic transformation (odds ratio, 4.9), as well as to other nonmyeloid malignancies.[17] Recently, a retrospective study of 208 patients (106 with polycythemia vera and 102 with essential thrombocythemia) with NGS data available demonstrated a higher probability of leukemic transformation in patients harboring the DNMT3A, SRSF2, and IDH1/2 mutations.[18]
In primary myelofibrosis, low JAK2 V617F allele burden has been associated with worse OS. Among 127 JAK2 V617F–positive patients with primary myelofibrosis, the lowest quartile of allele burden had significantly shorter survival compared with other quartiles; this was also seen in JAK2 wild-type patients. Worse outcome was mainly attributed to systemic infections.[19]
Evaluation of driver mutation status is of benefit in assessing prognosis in patients with primary myelofibrosis. In a study of 428 myelofibrosis patients, the median survival in JAK2-, MPL-, and CALR-mutated patients was 5.9, 9.9, and 15.9 years, respectively. Importantly, the worst prognosis was in patients without mutations in these three genes, the so-called “triple-negative” group, with a median survival of only 2.3 years and a higher risk of leukemic transformation.[20] The type of CALR mutation also appears to have distinct prognostic relevance. In a study of 358 primary myelofibrosis patients, those with a type 1 CALR mutation had significantly longer survival when compared with patients who had either JAK2 or type 2 CALR mutations. However, this survival difference disappeared when the Dynamic International Prognostic Scoring System (DIPSS)-Plus score and other mutations were added into the multivariate analysis.[7]
Other mutations have also been shown to have prognostic relevance in primary myelofibrosis. In a study of 879 patients, ASXL1, SRSF2, and EZH2 predicted shorter survival; however, only mutated ASXL1 retained its prognostic significance even when the DIPSS score was included in the analysis. Additionally, leukemia-free survival was negatively impacted by IDH1/2, SRSF2, and possibly ASXL1.[4] A separate study identified the triple-negative and CALR-nonmutated/ASXL1-mutated genotypes as high-risk molecular signatures associated with the worst median survival, 2.5 and 2.3 years, respectively.[21] Taken together, these data identify a high–molecular risk category based on the presence of ASXL1, EZH2, SRSF2, or IDH1/2. In a study of 797 patients with primary myelofibrosis, the presence of more than one high-risk mutation also predicted worse OS and shorter leukemia-free survival when compared with patients with no prognostically detrimental mutations.[22] Other mutations may also share prognostic importance in MPNs. In a series that included 197 patients with polycythemia vera, essential thrombocythemia, or primary myelofibrosis, the presence of TP53 and TET2 were independently associated with lower OS and shorter leukemia-free survival.[23]
Other Myeloid Malignancies: Prognosis
In chronic myelomonocytic leukemia, nonsense/frameshift mutations involving ASXL1 appear to negatively impact survival and improve prognostic value when added to the Mayo chronic myelomonocytic leukemia prognostic model.[24,25] SETBP1 mutations may affect OS, although results are inconsistent.[26] Mutations in RUNX1, on the other hand, do not affect OS but have been associated with shorter leukemia-free survival.[27]
CSF3R is frequently mutated in patients with chronic neutrophilic leukemia, which, as mentioned previously, is important diagnostically.[5,6] Other pathogenic mutations have been observed in chronic neutrophilic leukemia, including CALR, SETBP1, and JAK2 V617F, with unclear prognostic significance.[28] In a series of 14
CSF3R-mutated patients with chronic neutrophilic leukemia, ASXL1 was present in 57% and associated with shortened survival in multivariate analysis.[29]
In patients with systemic mastocytosis, the presence of mutated TET2 is associated with more aggressive forms of the disease, and in a subset of patients with 2008 WHO-defined systemic mastocytosis with associated clonal hematologic non–mast cell diseases, the presence of mutated ASXL1 negatively impacted OS.[30] Additionally, when NGS was applied to patients with systemic mastocytosis, OS was decreased when mutations other than in KIT D816V were observed, most frequently in TET2, SRSF2, ASXL1, CBL, and RUNX1.[31]
Myeloproliferative Neoplasms: Treatment Implications
Primary myelofibrosis
The only approved therapy, ruxolitinib, a JAK1/JAK2 inhibitor, significantly improves splenomegaly and symptom burden in the majority of treated myelofibrosis patients.[32] This clinical benefit has been shown to be irrespective of JAK2 mutation status or the presence of prognostically detrimental mutations (ASXL1, EZH2, SRSF2, IDH1/2).[33] However, when NGS was applied to samples obtained from 95 patients in a phase I/II trial of ruxolitinib, the number of mutations was inversely correlated with spleen response (patients with ≤ 2 mutations had ninefold higher odds of a spleen response than those with ≥ 3 mutations). Additionally, patients with three or more of these mutations had shorter time to treatment discontinuation and shorter OS.[34] The lack of JAK2 V617F does not preclude benefit from ruxolitinib treatment, although patients with a higher mutation burden (≥ 3 mutations) likely have more aggressive disease with worse outcomes even with ruxolitinib treatment.
There is a paucity of data on how mutation status affects hematopoietic stem cell transplantation outcomes. It has been proposed that patients with high-risk mutations, such as ASXL1 or triple-negative disease, should proceed to hematopoietic stem cell transplantation earlier than patients whose high-risk status has been clinically determined and who lack these molecular abnormalities.[35,36] This has not yet been validated in a prospective study.
CML
The cornerstone of CML treatment involves either a first-generation BCR-ABL1 tyrosine kinase inhibitor (TKI) (imatinib) or one of the more potent second-generation TKIs (dasatinib, nilotinib).[37] Importantly, the T315I mutation occurring in the ABL kinase domain of BCR-ABL1 confers imatinib resistance in CML.[38] Second-generation TKIs are also ineffective in patients harboring this specific mutation, as demonstrated in phase I and II clinical trials for dasatinib,[39,40] nilotinib,[41] and bosutinib.[42] Ponatinib is the only available TKI effective against the T315I mutation. In the phase II PACE trial, 449 patients with chronic, acute, or blast-phase CML or BCR-ABL1–positive acute lymphoblastic leukemia resistant to or intolerant of prior TKI therapy were treated with ponatinib, 45 mg once daily. Of those treated, 128 patients harbored the T315I mutation. Patients in all subgroups experienced cytogenetic and molecular responses; in the 270 patients with chronic-phase CML, 56% had a major cytogenetic response at 12 months, including 70% of those with the T315I mutation.[43] This study led to US Food and Drug Administration (FDA) approval of ponatinib for CML and BCR-ABL1–positive acute lymphoblastic leukemia.[44] However, arterial thrombotic events were a treatment-related adverse event observed with ponatinib in the PACE trial, at rates of 2.2%, 0.7%, and 1.6% for cardiovascular, cerebrovascular, and peripheral vascular disease, respectively.[43] This information, combined with postmarketing monitoring, led to an FDA suspension of sales in October 2013, although the suspension was lifted shortly afterwards, following implementation of new safety measures by the manufacturer.[45] Two trials (ClinicalTrials.gov identifiers: NCT02467270 and NCT02627677) are currently investigating a reduced dose of ponatinib to optimize its risk/benefit profile. Despite safety concerns, ponatinib represents an efficacious alternative (or bridge) to hematopoietic stem cell transplantation in patients with CML harboring the T315I mutation.
Chronic neutrophilic leukemia
In chronic neutrophilic leukemia, frameshift or truncating mutations of the cytoplasmic tail of CSF3R (S783fs mutation) produce a downstream increase in tyrosine kinase nonreceptor 2 and an Src family kinase.[5,46] In vitro, this mutation confers sensitivity to dasatinib. On the other hand, the presence of proximal mutations (eg, T618I) leads to increases in JAK signaling activity, which demonstrates in vitro sensitivity to ruxolitinib. This has also shown clinical benefit in a report of a single patient with chronic neutrophilic leukemia carrying this proximal mutation.[5,47]
Systemic mastocytosis
A mutation in KIT, most commonly D816V, is a molecular hallmark of systemic mastocytosis and is present in nearly all patients.[48] Unfortunately, this mutation confers resistance to imatinib treatment. Imatinib is currently the only agent that the FDA has approved for use in the treatment of systemic mastocytosis, specifically for non-D816V aggressive systemic mastocytosis.[49] Other TKIs may also be effective in this setting; dasatinib, for example, has shown preclinical activity and clinical efficacy in a small series of patients with systemic mastocytosis.[50] In a large phase II study of 33 patients with systemic mastocytosis, 85% of whom harbored the KIT D816V mutation, the overall response rate was 33%. However, most of the improvement was symptomatic, with only 2 patients achieving complete response.[51] Midostaurin has shown clinical activity in both wild-type and D816V-mutated systemic mastocytosis. In 2016, an international, multicenter, open-label phase II study examined the safety, efficacy, and patient-reported outcomes of midostaurin in 89 patients with advanced systemic mastocytosis. The patients were categorized as having aggressive systemic mastocytosis, systemic mastocytosis with an associated hematologic neoplasm, or mast cell leukemia. Response rates varied between 50% and 75% among the three groups, and response was seen among patients who were positive and negative for KIT D816V mutation. The median OS was significantly longer in responders than in nonresponders (44.4 months vs 15.4 months). Overall, midostaurin was shown to reverse organ damage, decrease splenomegaly and bone marrow mast cell burden, and improve patient quality of life.[52] Based on these promising results, this drug was approved by the FDA for the treatment of aggressive systemic mastocytosis, systemic mastocytosis with associated hematologic neoplasm, and mast cell leukemia in April 2017.[53]
Clonal Hematopoiesis of Indeterminate Potential
As genomic information is becoming increasingly relevant, there is heightened interest in novel assays to detect a variety of alterations. Recently, a novel comprehensive genomic profiling assay of DNA and RNA became available. It is able to detect a variety of clinically relevant genetic alterations (substitutions, insertions/deletions, copy number alterations, and a wide range of gene fusions) in hematologic malignancies.[54] However, with improvements in the quality and availability of mutation profiling, additional mutations are being discovered whose clinical relevance is unclear. One example of this is clonal hematopoiesis of indeterminate potential (CHIP). This newly coined entity is best defined as the presence of somatic mutations in hematologic malignancy–associated genes in blood or bone marrow cells in the absence of cytopenias or hematologic malignancy, or in patients with cytopenias that do not meet the strict criteria for WHO-defined myelodysplastic syndromes. In a patient with unexplained cytopenias, mutation profiling can provide diagnostic assistance (Figure 3). The mutated allele frequency must be > 2% to qualify for the CHIP diagnosis. Compared with myelodysplastic syndromes, CHIP is associated with longer survival and normal blood counts in most cases.[55] The most commonly mutated genes appear to be TET2 and DNMT3A, and the mutations are highly age-correlated.[56] The rate of progression to acute myeloid leukemia is low, at 0.5% to 1% per year, similar to the rate of progression in patients with monoclonal gammopathy of undetermined significance, although higher than in those without known mutations. Guidelines are currently nonexistent for the management of patients in this category. It is proposed that patients in whom mutations are discovered undergo routine surveillance, as in monoclonal gammopathy of undetermined significance, if hematologic abnormalities are present. For clinicians, the counseling and management of patients with CHIP will be an ongoing challenge, but the potential for early intervention in some patients is promising.
As mutation profiling becomes more widespread, it is essential to consider the financial implications of routine testing. The costs of high-throughput gene sequencing, such as NGS, continue to decline and currently cost approximately $200 to $1,000 per sample.[57] Moreover, payers may vary the level of coverage based on clinical circumstances. Poor reimbursement in many cases is likely based on the misconception that these tests are primarily for research purposes and remain of unproven benefit for direct patient care.[58] However, as noted above, genetic assessments have the potential to guide prognosis, therapy, and hematopoietic stem cell transplantation decision making. This personalized genomic information may enable clinicians to provide more effective and high-value care, surpassing the initial costs of molecular testing. Moving forward, payers may be more likely to increase reimbursement for molecular testing as our understanding of mutation profiling in myeloid malignancies expands.
Conclusion
Mutation profiling for patients with myeloid malignancies is already commercially available and widely included in the evaluation and management of many patients in the community. With genomics-directed precision oncology for our patients with myeloid malignancies still in its infancy, the challenge for the practitioner will be in staying updated on clinically relevant advances.
Financial Disclosure:Dr. Rampal serves as a consultant for Agios, Incyte, and Jazz Pharmaceuticals. The other authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-405.
2. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054-61.
3. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379-90.
4. Vannucchi AM, Lasho TL, Guglielmelli P, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861-9.
5. Maxson JE, Gotlib J, Pollyea DA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368:1781-90.
6. Pardanani A, Lasho TL, Laborde RR, et al. CSF3R T618I is a highly prevalent and specific mutation in chronic neutrophilic leukemia. Leukemia. 2013;27:1870-3.
7. Tefferi A, Lasho TL, Finke C, et al. Type 1 vs type 2 calreticulin mutations in primary myelofibrosis: differences in phenotype and prognostic impact. Leukemia. 2014;28:1568-70.
8. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472-6.
9. Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110:1092-7.
10. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874-81.
11. Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574-9.
12. Rotunno G, Pacilli A, Artusi V, et al. Epidemiology and clinical relevance of mutations in post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a study on 359 patients of the AGIMM group. Am J Hematol. 2016;91:681-6.
13. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117:5857-9.
14. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123:1552-5.
15. Elala Y, Lasho TL, Gangat N, et al. Driver mutations and prognosis in 502 patients with essential thrombocythemia. Blood. 2015;126:1599.
16. Tefferi A, Lasho TL, Finke C, et al. Targeted next-generation sequencing in polycythemia vera and essential thrombocythemia. Blood. 2015;126:354.
17. Hernandez-Boluda JC, Pereira A, Cervantes F, et al. A polymorphism in the XPD gene predisposes to leukemic transformation and new nonmyeloid malignancies in essential thrombocythemia and polycythemia vera. Blood. 2012;119:5221-8.
18. SenÃn A, Fernández C, Bellosillo B, et al. Role of non-driver mutations and JAK2V617F allele burden in myelofibrotic and acute myeloid transformation of patients with polycythemia vera and essential thrombocythemia. Blood. 2016;128:1952.
19. Guglielmelli P, Barosi G, Specchia G, et al. Identification of patients with poorer survival in primary myelofibrosis based on the burden of JAK2V617F mutated allele. Blood. 2009;114:1477-83.
20. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507-13; quiz 615.
21. Tefferi A, Lasho TL, Finke CM, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472-7.
22. Guglielmelli P, Lasho TL, Rotunno G, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia. 2014;28:1804-10.
23. Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123:2220-8.
24. Patnaik MM, Padron E, LaBorde RR, et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia. 2013;27:1504-10.
25. Patnaik MM, Itzykson R, Lasho TL, et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia. 2014;28:2206-12.
26. Laborde RR, Patnaik MM, Lasho TL, et al. SETBP1 mutations in 415 patients with primary myelofibrosis or chronic myelomonocytic leukemia: independent prognostic impact in CMML. Leukemia. 2013;27:2100-2.
27. Itzykson R, Kosmider O, Renneville A, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31:2428-36.
28. Elliott MA, Tefferi A. Chronic neutrophilic leukemia 2016: update on diagnosis, molecular genetics, prognosis, and management. Am J Hematol. 2016;91:341-9.
29. Elliott MA, Pardanani A, Hanson CA, et al. ASXL1 mutations are frequent and prognostically detrimental in CSF3R-mutated chronic neutrophilic leukemia. Am J Hematol. 2015;90:653-6.
30. Damaj G, Joris M, Chandesris O, et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS One. 2014;9:e85362.
31. Jawhar M, Schwaab J, Schnittger S, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016;30:136-43.
32. Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787-98.
33. Guglielmelli P, Biamonte F, Rotunno G, et al. Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT-II study. Blood. 2014;123:2157-60.
34. Patel KP, Newberry KJ, Luthra R, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015;126:790-7.
35. Kroger N, Giorgino T, Scott BL, et al. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis. Blood. 2015;125:3347-50; quiz 64.
36. Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1262-71.
37. Jabbour E. Chronic myeloid leukemia: first-line drug of choice. Am J Hematol. 2016;91:59-66.
38. Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117-25.
39. Soverini S, Colarossi S, Gnani A, et al. Resistance to dasatinib in Philadelphia-positive leukemia patients and the presence or the selection of mutations at residues 315 and 317 in the BCR-ABL kinase domain. Haematologica. 2007;92:401-4.
40. Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114:4944-53.
41. Lange T, Ernst T, Gruber FX, et al. The quantitative level of T315I mutated BCR-ABL predicts for major molecular response to second-line nilotinib or dasatinib treatment in patients with chronic myeloid leukemia. Haematologica. 2013;98:714-7.
42. Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567-76.
43. Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075-88.
44. US Food and Drug Administration. FDA approves Iclusig to treat two rare types of leukemia. https://wayback.archive-it.org/7993/20170112023921/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm332252.htm. Accessed April 5, 2018.
45. FDA Drug Safety Communication. FDA requires multiple new safety measures for leukemia drug Iclusig; company expected to resume marketing. https://www.fda.gov/Drugs/DrugSafety/ucm379554.htm. Accessed April 5, 2018.
46. Futami M, Zhu QS, Whichard ZL, et al. G-CSF receptor activation of the Src kinase Lyn is mediated by Gab2 recruitment of the Shp2 phosphatase. Blood. 2011;118:1077-86.
47. Dao KH, Solti MB, Maxson JE, et al. Significant clinical response to JAK1/2 inhibition in a patient with CSF3R-T618I-positive atypical chronic myeloid leukemia. Leuk Res Rep. 2014;3:67-9.
48. Erben P, Schwaab J, Metzgeroth G, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol. 2014;93:81-8.
49. US Food and Drug Administration. FDA approves imatinib mesylate (Gleevec) as a single agent for the treatment of multiple indications. 2006.
50. Purtill D, Cooney J, Sinniah R, et al. Dasatinib therapy for systemic mastocytosis: four cases. Eur J Haematol. 2008;80:456-8.
51. Verstovsek S, Tefferi A, Cortes J, et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin Cancer Res. 2008;14:3906-15.
52. Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530-41.
53. US Food and Drug Administration. FDA approves new combination treatment for acute myeloid leukemia. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm555778.htm. Accessed April 5, 2018.
54. He J, Abdel-Wahab O, Nahas MK, et al. Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood. 2016;127:3004-14.
55. Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9-16.
56. Buscarlet M, Provost S, Zada YF, et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130:753-62.
57. Duncavage EJ, Tandon B. The utility of next-generation sequencing in diagnosis and monitoring of acute myeloid leukemia and myelodysplastic syndromes. Int J Lab Hematol. 2015;37(suppl 1):115-21.
58. Yang F, Press RD. Next-generation sequencing multi-gene mutation panels in myeloid malignancies. http://www.hematology.org/Thehematologist/Mini-Review/5496.aspx. Accessed April 5, 2018.
59. Levine RL, Loriaux M, Huntly BJ, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106:3377-9.
60. Jones AV, Kreil S, Zoi K, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162-8.
61. Lippert E, Boissinot M, Kralovics R, et al. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood. 2006;108:1865-7.
62. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779-90.
63. Scott LM, Beer PA, Bench AJ, et al. Prevalance of JAK2 V617F and exon 12 mutations in polycythaemia vera. Br J Haematol. 2007;139:511-2.
64. Lasho TL, Elliott MA, Pardanani A, Tefferi A. CALR mutation studies in chronic neutrophilic leukemia. Am J Hematol. 2014;89:450.
65. Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767-73.
66. Daver N, Strati P, Jabbour E, et al. FLT3 mutations in myelodysplastic syndrome and chronic myelomonocytic leukemia. Am J Hematol. 2013;88:56-9.
67. Cancer Genome Atlas Research Network, Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059-74.
68. Boultwood J, Perry J, Pellagatti A, et al. Frequent mutation of the polycomb-associated gene ASXL1 in the myelodysplastic syndromes and in acute myeloid leukemia. Leukemia. 2010;24:1062-5.
69. Brecqueville M, Rey J, Bertucci F, et al. Mutation analysis of ASXL1, CBL, DNMT3A, IDH1, IDH2, JAK2, MPL, NF1, SF3B1, SUZ12, and TET2 in myeloproliferative neoplasms. Genes Chromosomes Cancer. 2012;51:743-55.
70. Ernst T, Chase AJ, Score J, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42:722-6.
71. Tefferi A, Pardanani A, Lim KH, et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia. 2009;23:905-11.
72. Stegelmann F, Bullinger L, Schlenk RF, et al. DNMT3A mutations in myeloproliferative neoplasms. Leukemia. 2011;25:1217-9.
73. Abdel-Wahab O, Pardanani A, Rampal R, et al. DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leukemia. 2011;25:1219-20.
74. Tefferi A, Jimma T, Sulai NH, et al. IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: clinical evidence for leukemogenic collaboration with JAK2V617F. Leukemia. 2012;26:475-80.
75. Greaves WO, Verma S, Bisrat T, et al. TP53 mutation is rare in primary myelofibrosis. Leuk Lymphoma. 2013;54:1552.
76. Lasho TL, Finke CM, Hanson CA, et al. SF3B1 mutations in primary myelofibrosis: clinical, histopathology and genetic correlates among 155 patients. Leukemia. 2012;26:1135-7.
77. Martinez-Aviles L, Besses C, Alvarez-Larran A, et al. Mutations in the RNA splicing machinery genes in myelofibrotic transformation of essential thrombocythaemia and polycythaemia vera. Br J Haematol. 2014;164:605-7.
78. Makishima H, Yoshida K, Nguyen N, et al. Somatic SETBP1 mutations in myeloid malignancies. Nat Genet. 2013;45:942-6.
79. Piazza R, Valletta S, Winkelmann N, et al. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nat Genet. 2013;45:18-24.
