Impressive Antitumor Activity With XELOX in Phase II Trial
BARCELONA, Spain-A large European, multicenter phase II trial of XELOX-capecitabine (Xeloda) and oxaliplatin (Eloxatin, investigational in the United States)-as first-line therapy for patients with metastatic colorectal cancer produced an objective response in 55% of patients.
BARCELONA, SpainA large European, multicenter phase II trial of XELOXcapecitabine (Xeloda) and oxaliplatin (Eloxatin, investigational in the United States)as first-line therapy for patients with metastatic colorectal cancer produced an objective response in 55% of patients.
Josep Tabernero, MD, Oncologia Medica, Vall d’Hebron Hospital, Barcelona, Spain, presented the results at the 38th Annual Meeting of the American Society of Clinical Oncology (abstract 531).
"The most important finding is that XELOX is a highly effective regimen with a good safety profile," Dr. Tabernero said. "And it is very convenient for the patient."
The XELOX regimen is a logical drug combination, said Christopher Twelves, MD, Cancer Research UK, Glasgow, Scotland. Dr. Twelves spoke at an industry-sponsored symposium held in conjunction with the Orlando ASCO meeting.
"These two molecules have clearly different modes of action, and we know from past experience that fluorouracil (5-FU)/leucovorin combined with oxaliplatin is a highly active regimen," Dr. Twelves said. "Xeloda is at least as effective as 5-FU and has the advantage of being taken orally. There is no overlap in major toxicities with oxaliplatin and Xeloda. The major toxicity with oxaliplatin is sensory neuropathy, which isn’t a feature of Xeloda. We also have preclinical data suggesting genuine synergy between fluoropyrimidines and oxaliplatin."
Study Protocol
The study involved 96 patients with metastatic colorectal cancer who were given a 2-hour intravenous infusion of oxaliplatin 130 mg/m² on day 1 and oral capecitabine 1,000 mg/m² bid on days 1 through 14, followed by a 7-day rest. Treatment continued for 11 cycles in patients with stable disease or tumor response, or until disease progression.
About half of the patients (47%) received the full doses of both drugs throughout their treatment. Eighteen percent of patients required a lower dose of capecitabine, 14% required less oxaliplatin, and 22% required a lower dose of both drugs.
The median number of cycles received was 10 (range, 1 to 23). "One of the first intimations we get of a regimen’s activity is the number of cycles received," Dr. Twelves said. "The fact that patients were able to continue this treatment for a prolonged period of time is a clear indication that it is effective and well tolerated."
Said Dr. Tabernero: "The patients understand that this is for their lives, that this is chemotherapy they should take. We have not had a problem with compliance, which was more than 95%."
Eligible patients were 18 to 75 years old and had histologically confirmed, measurable metastatic colorectal cancer, a Karnofsky performance status greater than 70, and a life expectancy of at least 3 months. Patients who had received chemotherapy for metastatic disease; had prior treatment with capecitabine, oxaliplatin, or irinotecan (Camptosar); or had been given any chemotherapy in the previous 6 months were excluded. The 196 patients in the study had a median age of 64 (range, 34 to 79) and Karnofsky scores ranging from 80 to 100.
Disease characteristics were as follows: colon tumors (63% of patients); rectal cancers (33%, with unspecified tumor site in 4 patients); single metastatic site (46%); more than one metastatic site (54%); liver metastases (78%); lung metastases (32%). About one quarter of the patients had received prior adjuvant chemotherapy.
Thirty of the 96 patients (31%) discontinued oxaliplatin because of toxicity, but they continued on the capecitabine. These patients were included in the data analyses along with those who remained on the XELOX combination.
Tumor response was assessed at baseline, after three cycles (9 weeks), and every 6 weeks thereafter.
Outcomes
In this intention-to-treat analysis, with a minimum follow-up of 12 months, 55% of patients experienced an objective response and 32% stable disease. Of the 53 responding patients, 2 had a complete response and 51 a partial response.
Disease stability lasted more than 3 months in all 31 patients achieving this outcome. "So this wasn’t just a single snapshot where the patient was progressing but hadn’t quite yet reached the 25% increase in the size of their tumor that we define as progression. This was genuine stabilization of the disease for a useful period of time," Dr. Twelves commented.
Progressive disease occurred in 6% of patients, and 6% discontinued treatment early.
Dr. Tabernero reported that progression-free survival was estimated at 7.6 months. Twenty months after the study began and 12 months after the last patient enrolled, 13 patients have not experienced disease progression, and three patients continue to receive treatment.
Fifty-seven patients are still alive. Median overall survival is currently greater than 16 months but has not been reached. Investigators expect median survival to increase over time. The 12-month survival currently stands at 72%.
Subgroup Analysis
When the researchers looked at subgroups (prior adjuvant chemotherapy, liver or lung metastases, Karnofsky performance status 80 or higher, or age above or below 60 years), the high response rate was maintained, with all groups achieving a rate of 50% or higher.
Dr. Twelves pointed out that while investigators are taught to be wary of subgroup analyses, in a phase II trial with nearly 100 patients, many of the subgroups are as large as most individual phase II trials would be.
Safety Data
Investigators evaluated safety in all patients who received treatment. The safety profile was similar to previous studies involving XELOX, Dr. Tabernero said. Dr. Twelves noted that it compares favorably to the safety profile of FOLFOX4 (see table below), which some consider the new standard of care in metastatic colorectal cancer. FOLFOX4 consists of oxaliplatin and infusional 5-FU/leucovorin.
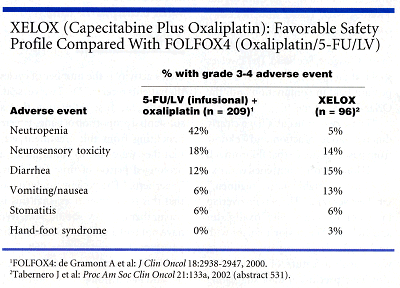
The most common adverse reactions to XELOX were sensory neuropathy, 81% for all grades and 14% for grades 3-4, and gastrointestinal disturbances, with 15% experiencing grade 3-4 diarrhea and 13% grade 3-4 nausea/vomiting. Three patients had grade 3 hand-foot syndrome.
Treatment-related grade 4 adverse effects occurred in only five patients. One patient with a prior history of pulmonary fibrosis died of respiratory failure. The death has been attributed to the study treatment, presumably the oxaliplatin.
"This regimen seems to be as effective and safe as FOLFOX," Dr. Tabernero said, "and it’s clearly more convenient."
Phase III evaluations comparing XELOX with FOLFOX4 are planned. "It’s very important to have clinical trials to definitely say that XELOX is equally safe and effective," Dr. Tabernero concluded. "FOLFOX is the standard in Europe, and oxaliplatin is fast tracked at the FDA."