Improving Palliative and Supportive Care in Cancer Patients
Twenty years of research in controlling symptoms such as pain andnausea have shown persistent suboptimal performance by the US oncologysystem. The data suggest that some of the tools of palliative careprograms can improve physical symptoms of seriously ill patients at acost society can afford. To fix these problems will require recognitionof the symptoms or concerns, a system such as an algorithm or careplan for addressing each, measurement of the change, and accountabilityfor the change. Symptom assessment scales such as the EdmontonSymptom Assessment Scale or Rotterdam Symptom Check List work tomake symptoms manifest. Listing symptoms on a problem list is a necessarystep in addressing them. Physical symptoms such as pain can beimproved by use of computer prompts, algorithms, dedicated staff time,team management, or combinations of these strategies. Less concreteproblems such as medically appropriate goal-setting, integrating palliativecare into anticancer care sooner, and informing patients aboutthe benefits and risks of chemotherapy near the end of life require morecomplex solutions. We review what is known about symptom control inoncology, how and why some programs do better, and make suggestionsfor practice. Finally, we suggest a practical plan for using symptomassessment scales, listing the problems, and managing them accordingto algorithms or other predetermined plans.
Twenty years of research in controlling symptoms such as pain and nausea have shown persistent suboptimal performance by the US oncology system. The data suggest that some of the tools of palliative care programs can improve physical symptoms of seriously ill patients at a cost society can afford. To fix these problems will require recognition of the symptoms or concerns, a system such as an algorithm or care plan for addressing each, measurement of the change, and accountability for the change. Symptom assessment scales such as the Edmonton Symptom Assessment Scale or Rotterdam Symptom Check List work to make symptoms manifest. Listing symptoms on a problem list is a necessary step in addressing them. Physical symptoms such as pain can be improved by use of computer prompts, algorithms, dedicated staff time, team management, or combinations of these strategies. Less concrete problems such as medically appropriate goal-setting, integrating palliative care into anticancer care sooner, and informing patients about the benefits and risks of chemotherapy near the end of life require more complex solutions. We review what is known about symptom control in oncology, how and why some programs do better, and make suggestions for practice. Finally, we suggest a practical plan for using symptom assessment scales, listing the problems, and managing them according to algorithms or other predetermined plans.
TABLE 1

Symptom Control and Important Care Planning Issues in Oncology
Oncology health-care professionals pride themselves on taking the best possible care of their patients, and the American Society of Clinical Oncology and Oncology Nursing Society have been emphasizing the care of the total patient for years. Foley first published her landmark article on cancer pain treatment in 1985, describing deficiencies and remedies.[1] Oncologists recognized over a decade ago that pain and other symptom control in their own practices was not optimal.[2,3] Unfortunately, pain control does not seem to have improved as much as the recognition of poor performance. The Institute of Medicine noted that the quality of all cancer care is too often not optimal.[4] While much of the emphasis in quality improvement has been with outcomes such as survival and complication rates,[5,6] supportive and palliative care has remained deficient for many cancer patients in a number of areas,[7,8] as shown in Table 1. Like an indolent lymphoma, the problem keeps surfacing.
There are remarkably few data on relief of other symptoms such as fatigue, dyspnea, delirium, agitation, or how well oncologists help patients clarify wishes at the end of life, but it is a recurring complaint from health-care professionals that come to our center to learn how to do palliative care. We know that only half of all patients receiving cisplatin-containing regimens receive appropriate delayed-emesis regimens, even though adherence to antiemetic guidelines improves outcomes.[9,10] Fewer than 20% of on cology inpatients have documented "do/do not resuscitate" orders, a number that has not increased despite 20 years of efforts to increase public awareness about advance directives.[11] We know that 20% or more of Medicare patients are being treated with chemotherapy within a month of their death,[12,13] patients are being referred later and later to hospice,[14] and patients who do not know or overestimate their prognosis are more likely to die in the hospital or intensive care unit.[15,16] We have listed some of the known areas for symptom improvement in Table 1.[2,3,9,17-24]
If symptom relief is good medicine, patients want it, society wants it, and the tools are available, why has it not been routinely accomplished? The reasons for these findings are varied, but issues such as symptom awareness, symptom assessment, provider knowledge, provider preference, clinical autonomy, and provider time burden likely play some role in these clinical outcomes.[25,26] Recent reviews have noted some of the substantial patient, family, provider, and payer barriers to enhancing symptom control and palliative care especially at the end of life,[27] so we should not expect rapid and miraculous change. Some cancer centers have begun programs to fully integrate symptom control into their programs,[28] but this has not trickled down to the everyday practice reported to us when cancer centers come to learn how to set up palliative care programs.
In this review, we will cover some practical attempts to improve care, much of which is based on our experience as a Palliative Care Leadership Center charged with helping cancer centers integrate symptom control into the care of their patients. To do so will require at least the following: (1) recognition that care is not optimal now; (2) clinical trial evidence that outcomes can be improved; (3) ways to make symptoms easily visible; (4) and methods to improve practitioner behavior. We will concentrate on what oncologists can do to improve care in their offices and practices today.
Palliative and Supportive Care Is Suboptimal
There are no national data on symptom control, but all the available practical evidence and the evidence listed above suggests that care really is not optimal. For instance, the Joint Commission on Accreditation of Healthcare Organizations made pain the fifth vital sign, in an attempt to make hospitals at least measure pain and make it visible. The National Quality Forum recently created a national task force to make auditable guidelines for symptom control and end-of-life care. We have listed some of the most easily recognizable deficiencies above, but there is needed work in patient communication, patient recognition and acceptance of the end of life, recognition of spiritual needs, integration of family meetings into difficult goal-setting sessions, and management of these tasks along with other professional duties, to name a few.
In the coming years, oncologists will be expected to provide more proof that therapy is both effective and affordable. This has come to the fore with the expensive new medicines such as oxaliplatin (Eloxatin), cetuximab (Erbitux), bevacizimab (Avastin), irinotecan (Camptosar), gefitinib (Iressa), granulocyte colony-stimulating factor (filgrastim, [Neupogen]), pegylated granulocyte colony-stimulating factor (pegfilgrastim [Neulasta]), epoetin alfa (Epogen, Procrit), and darbepoetin (Aranesp).[29] Any new ventures will be expected to show that they can fit into increasingly tight budgets. Increased supportive care and palliative care will be subjected to the same scrutiny as new medications.
Evidence That Outcomes Can Be Improved
Clinical Outcomes
FIGURE 1
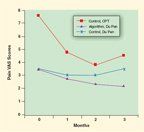
Effect of Interventions on Pain in Cancer Patients
Several trials have shown that oncology cancer patients can achieve better pain and symptom outcomes in regular practice. In a trial conducted in community oncology practices in 1997, Du Pen and colleagues randomized patients to two treatment arms.[30] Group 1 received standard care from their usual oncology care practitioners and treatments; group 2 was interviewed by a nurse to ascertain pain scores, and the pain was then managed by the team according to an algorithm based on the Agency for Health Care Policy and Research (AHCPR, now the Agency for Healthcare Quality Research) guidelines.[31] The reduction in pain scores is shown in Figure 1. The standard group showed no improvement, even though the practitioners knew the study was ongoing in their offices and the results would be reported. The intervention group had a significant (25%-40%) reduction in pain, which was sustained as long as the trial lasted.
In the Cancer Pain Trial,[32] the control group (Control CPT in Figure 1) had a 39% reduction in pain scores. The intervention in the control group included comanagement by the oncologist and a pain specialist, using recommended algorithms, instead of by the oncologist alone. The control group had changes in the dose and type of opioids, additional drugs such as neuroleptics, and better management of side effects. Since two separate pain scores over 5 out of 10 were required to enter the trial, it seems highly unlikely that continued management by the oncologist alone would have been effective.
Higginson has systematically reviewed the evidence for palliative care programs and found that although the quality of the studies varies widely, symptoms can be improved.[33] For pain alone, hospital-based palliative care teams had an effect size of 0.60, or a 60% reduction in pain scores, and an overall effect size of 0.22, or a 22% reduction in other symptoms. For a patient with a visual analog score of 8/10, the new score would be only 3.2 after the intervention-very clearly important.[34,35] The effectiveness of palliative care and hospice consultation teams in all settings was also striking, resulting in a reduction in patient pain scores (odds ratio [OR] = 0.38, 95% confidence interval [CI] = 0.23-0.64) and other symptoms (OR = 0.51, CI = 0.30-0.88), as well as a nonsignificant trend toward benefits in terms of patient satisfaction and therapeutic interventions. Data regarding home deaths were equivocal. Such comparisons were limited by the quality of the research, but all the available data, taken together, show a positive effect.
Miaskowski and colleagues recently reported on the success of a patient education program in reducing cancer pain scores.[36] The intervention group was given specific education, taught how to use a pillbox, and given "written instructions on how to communicate with their physician about unrelieved pain and the need for changes in their pain medication." The intervention group also had two home visits and added telephone calls for coaching. This involvement of patients lowered average pain scores by 32.5%, compared with an increase of 1.9% in the control group.
FIGURE 2

Symptom Improvement With Treatment by Algorithm
Solid evidence shows that hospitalbased consultation teams and inpatient units can reduce symptoms for inpatients. At the University of Texas M. D. Anderson Cancer Center, investigators noted a substantial reduction in symptoms after a palliative care consultation (using the Edmonton Symptom Assessment Scale to gauge symptoms, predetermined treatments for common symptoms, and expert attendings). Pain scores were reduced by 44%, nausea by 41%, fatigue by 39%, and dyspnea by 38%.[37]
As part of a quality assurance program to assess our use of algorithms, we tracked symptom scores on all patients admitted to the Thomas Palliative Care Unit during a 1-month period. As shown in Figure 2, all the measured symptoms were reduced. Although this was not a high-powered study, the results are strikingly similar to those produced in over 2,500 patients by the Mount Sinai Palliative Care Consultation Service (data available at www.capc.org).
Financial Outcomes
TABLE 2

Representative Data Showing That Improved Supportive or Palliative Care Can Improve Overall Health-Care Costs
The available data suggest that more attention to symptom control and care planning can reduce healthcare costs (Table 2).[37-41] Many of these findings, however, have appeared in business-oriented publications and are not readily accessible to practicing oncologists. In addition, much of the data is proprietary and sensitive, in that health-care plans do not want members to think they are reducing services or switching away from active treatment just to save money. Hospice cannot be expected to save enough money that there will be room to give more expensive care. In fact, the newer data suggest that cancer patients who choose hospice probably conserve only a small amount-about 10% of the costs for patients with aggressive cancer, and some minor cost savings for younger Medicare patients with cancer.[42] Much of the available data and concordant evidence is summarized on the Center to Advance Palliative Care website (www.capc.org) or available from our data analyst and business manager, J. Brian Cassel, PhD (jbcassel@hsc.vcu.edu).
FIGURE 3

Palliative Care Unit and Costs
At our own center, we have studied the financial outcomes for both scientific publication[38] and the business community,[43] and the costs are consistently about 50% of usual care for patients who have died in the hospital (Figure 3). This includes matched case controls of patients who died on the service and in the rest of the hospital, cohort series, and patients transferred from other units. The health system has "cost avoidance" of at least $1 million annually due the transfer of patients from high-cost units to the lower-cost unit. In addition, bed availability, especially in the intensive care units, has improved.
The impact of palliative care on the individual physician and practice is more difficult to define. There is no procedure associated with palliative care, and the time spent in difficult family meetings, goal setting, and so forth has not been as well reimbursed as chemotherapy.[44] Very little has actually been published about the amount of time needed, or actually spent, in day-to-day oncology care. Our group has shown that the amount of time needed to have a "do not resuscitate" discussion with a cancer patient is only 12 minutes or less, and should be includable in most hospital or office visits.[45] Some of the limited available data suggest that physicians may be spending only 3 ± 3 minutes in the room of seriously ill hospitalized patients,[46] which is not enough time to have these discussions. More data are clearly needed on typical practice patterns and concerning whether the reimbursement is sufficient to cover the expense of more time-consuming practice.
This situation may be different with the use of costly supportive drugs, which is outside the realm of this review. While supportive care drugs such as epoetin alfa, darbepoetin, filgrastim, and the like may reduce toxicity and even have a small impact on survival, their high acquisition cost makes their cost-effectiveness a concern except in cases of clear-cut survival benefit.
Ways to Make Symptoms Visible
The Center to Advance Palliative Care has made it standard procedure for all of the Palliative Care Leadership Centers to use a standardized symptom assessment scale. These are all validated, reliable, easy-to-use instruments that assess multiple symptoms. We have recently begun, like many other centers, to use these in our everyday practice. All of these tools are readily available free of charge on the City of Hope Pain/Palliative Care Resource Center website (www.cityofhope.org), among others. Although initially reluctant to use these forms, we are convinced that they make symptoms visible, corroborating the published scientific data that suggest these forms highlight important issues.[47-49]
TABLE 3

Symptom Assessment Scale Used at Thomas Palliative Care Program
This type of symptom assessment scale should be familiar to oncologists. As part of a demonstration project, three questions were borrowed from the Rotterdam Symptom Checklist[49] to form a scale with which Medicare oncology patients could be assessed during their visit (Table 3). If the symptoms in question were assessed, and a plan documented in the chart to address them, the oncologist would be paid $130. (See www.cms.hhs.gov/media/press/release.asp?Counter=1245 for links to the demonstration project.)
It is possible to use these forms in everyday practice. It takes a few minutes to ask the questions in a standard way, record them on a form, and then transfer them to a graph. The creators of the Edmonton Symptom Assessment Scale note that the recorder should not just add another mark to the existing graph, as the answer will be too heavily influenced by prior results.[50]
FIGURE 4

Daily Note Incorporating Symptom Assessment Scale
According to local interpretation of the Medicare regulations, if the patient fills out the form, the time spent is not reimbursable; if the physician asks the questions, that is reimbursable and would readily dovetail with most review of systems procedures done at the bedside. However, practitioners are urged to consult with their local billing and documentation experts. Figure 4 shows one of our daily encounter forms with a symptom assessment scale; these are available for modification from Carrie Cybulski at the Thomas Palliative Care Program, ccybulski@mail1.vcu.edu. These forms also include space for documentation of multidisciplinary rounds and Family Conferences; although we cannot prove that this improves care, it makes intuitive sense that noticed problems are more likely to be addressed and fixed.
Ways to Change Physician Behavior
Much is known about how-and how not-to change physicians or health care provider behavior. Techniques such as lecture methods do not work,[51] although they are the most common approaches. Other approaches such as "academic detailing" (using the techniques of a pharmaceutical representative) appear to have more impact.[52] In this section, we review the available data on improving physician prescribing or practice in supportive and palliative care.
Clinical Practice Guidelines
Clinical practice guidelines[53] have been used to disseminate evidence-based clinical management recommendations to the medical community and to improve the overall quality of health-care delivery by ensuring consistent, standardized care for all patients with a given diagnosis. Unfortunately, guidelines alone have little effect on the practice habits of most clinicians; this may be due to ignorance of guideline content, interest in clinical autonomy, or simply inconsistent individual practice patterns. For example, despite national publication of guidelines for hematopoietic colony-simulating factor use in 1994 and 1996 (by the American Society of Clinical Oncology), physician practice surveys taken in 1994 (before initial guidelines publication) and 1997 continued to demonstrate significant variability in colony-stimulating factor use.[54,55]
Although extensive literature addresses the development of clinical practice guidelines, there is far less study in the area of implementation. Grol identified a variety of approaches to change clinical practice (educational, epidemiologic, marketing, social interaction, organizational change, and coercion) and described a cyclical process for proposing a needed change, identifying obstacles toward that change, and developing a plan to implement it.[56,57] Thus, the development of clinical practice guidelines by a national organization or governing body is a strategy that does not work (by itself) to influence physician practice.
TABLE 4

Predicting the Likelihood That Clinical Practice Guidelines Will Change Physician Practice
Grimshaw and Russell[58] (modified by Smith and Hillner[59]) reported a practical plan to improve effectiveness of clinical practice guidelines by development, dissemination, and implementation strategy. The strategies with the highest likelihood of success in changing behavior include those with internal development, specific educational intervention, a patient-specific reminder at the time of consultation, and individual accountability (Table 4). Although these strategies pertain to guideline compliance, they are a natural surrogate for implementing any standard care-sets developed within an institution.
Computerized Prompts
The quality of health-care delivery can be improved if appropriate point-of-service interventions (prompts and algorithms) are implemented. In a meta-analysis of 16 randomized, controlled trials, Shae and colleagues found that computerized clinical reminders (compared to control groups) improved preventive medicine services in areas such as vaccinations, breast and colon cancer screening, and cardiovascular risk reduction.[60] In other studies, adherence appears to be high in the ambulatory care setting and the inpatient setting when computerized clinical reminders are used, but there appears to be wide variability in physician performance.[61,62] We review here some practical studies in oncology.
• Erythropoietin Studies-Kralj and colleagues demonstrated that computerized clinical reminders in community practice settings improve the likelihood of erythropoietic drug prescription in patients with anemia.[63] This case control study assessed prescribing rates of erythropoietic agents by physicians in two community oncology practice settings over a 21-month period involving 11,644 physician-patient encounters. The intervention was a clinical reminder in real time through the electronic medical record (experimental group) triggered by patients presenting with a hemoglobin level less than 12 g/dL at any time during the 14 days before the clinic visit. The control group consisted of community physicians working through the same electronic record system without the pop-up reminder.
Erythropoietic drug prescription improved 14.2% in the experimental group during the intervention period (when physicians were subject to the computerized reminder) compared to the 4-month baseline period before the reminder was activated in the electronic system.[63] Interestingly, during the intervention period, erythropoietin prescribing rates at the control clinics declined by almost 16%. The difference in prescribing practices between the experimental and control groups (during the intervention period) was almost tripled. The multivariate logistic regression analysis model demonstrated the relative net impact of the intervention effect (clinical reminder) captured by the estimated coefficient of the interaction between time period and clinic. Results demonstrated that anemic patients in the experimental clinic were almost twice as likely (OR = 1.92, P = .008) to be treated with an erythropoietic agent.
Khatcheressian and colleagues documented substantial underuse of epoetin alfa for chemotherapy-associated anemia in their practice (hemoglobin < 10 g/dL) and attempted a clinic-wide solution.[64] After the prestudy chart review, the intervention included (1) specific educational interventions,(2) patient-specific clinical reminders at time of clinical encounter (point-of-service correction), wherein the nurse could invoke standing orders for epoetin alfa, and (3) shared responsibility for following the guideline (accountability). Nurses were encouraged to identify patients with chemotherapy-induced anemia during clinical encounters when they retrieved the blood count results, and recommend erythropoietic drug use to physicians at the point of service. A sample algorithm was used universally across the clinic that recommended a starting dose of 40,000 U/wk subcutaneously, with escalation as indicated.
During the preintervention period, 30 patients who received chemotherapy in the prior 2 months were identified; of these, 8 (27%) developed chemotherapy-induced anemia and only 4 (50%) were started on erythropoietic therapy.[64] In the postintervention period, 7 out of 35 patients (20%) developed chemotherapy-induced anemia; all 7 (100%) were treated with erythropoietin (P = .029). Based on retrospective interviews, it appears that a significant proportion of the success of this study is due to the diligence of the nursing staff in persistently identifying patients appropriate for treatment and making recommendations for erythropoietin use to the treating physician, as reported by Mertens et al.[9]
• HRT Study-The use of hormone replacement therapy (HRT) after randomized clinical trials showed the need to change prescribing practice has some analogy to oncology practice in that the problems must be recognized and corrected quickly. Computerized clinical reminders were employed in the Veterans Affairs (VA) hospital system to improve appropriate prescribing patterns for HRT after the Women's Health Initiative (WHI) study demonstrated an increased risk of cardiovascular events with such treatment.[65] This study was conducted to address several issues relating to incorporation of practice recommendations in physician practice, including (1) the escalating clinical information providers must "process and put into practice," (2) the ineffectiveness of guidelines or continuing medical education to influence behavior, (3) the perception that formulary changes impose on physician autonomy, and (4) the failure of implementation strategies due to unforeseen burdens on the time available for the physician–patient encounter. A three-part intervention was adopted (patient and physician education and computerized reminders as a "pharmacy alert" on the patient electronic record).
A unique aspect of this study was its focus on patient (as well as physician) education via personalized letters reporting on the WHI results, risks and benefits of HRT, and recommended discussion with the treating physicians regarding the best possible treatment course. The electronic alert was activated as a pop-up window once the provider accessed the electronic chart of a patient on HRT, thereby overcoming "a common barrier to action," in the words of the authors, "the need for the provider to identify all patients affected by the new information [guidelines]." This alert asked the provider to re-evaluate the need for HRT and generate an addendum to the alert in the form of action options including continuation or discontinuation of treatment at the current time or at some point in the future, thus encouraging definitive decisionmaking at the time of clinical encounter (point of service). Rather than being based on a strict algorithm, this system provides for individualized decision-making, preserves patient autonomy, and makes physicians ac accountable for their decisions.
The limitation of such an implementation system lies in the fact that it requires a totally electronic medical record system. In many institutions, patient management and treatment is possible without accessing a patient record in the computer information system (due to the availability of paper charts, audio dictation systems, and automated printing of lab results); computerized reminders in these settings are viewed as complementary to other interventions, not requisite or exclusive.
Algorithms and Nursing Staff
Treatment algorithms have proven useful in many clinical settings, facilitating the standardization of care for commonly encountered medical issues.
• Antiemetic Study-Mertens and colleagues[9] noted that only 25% of antiemetic prescriptions in their investigation met guidelines, and that too many patients experienced delayed chemotherapy-induced nausea and vomiting (Table 1). The solution demonstrated the importance of nonphysician health-care workers (ie, nurses and nurse practitioners) in improving physician compliance with guideline-based antiemetic prescription recommendations.
In their institution's experience, physician prescribing practice for the treatment of chemotherapy-induced nausea and emesis was not improved despite institutional development of clinical practice guidelines, guideline distribution, visiting expert lectures, and sharing of adherence data with clinicians. However, once patient outcomes (prevalence of delayed emesis) were shared with physicians, a program-wide adoption of antiemetic prescription writing by nurse practitioners (in compliance with institutional guidelines rather than individual physician preference) resulted in consistent and sustained improvement.[9] Barriers to identifying appropriate patients and implementing the correction at time of clinic encounter were overcome by the nurse practitioners in this study, as opposed to the computerized reminders in the VA study on HRT.
• Pain Study-Another study demonstrating the importance of nursing staff in invoking algorithms comes from a trial completed at community oncology practices in 1997. Du Pen and colleagues[30] randomized patients to two treatment arms: Group 1 received standard care and treatment from their usual oncology care practitioners; group 2 patients were interviewed by a nurse practitioner to ascertain pain scores. Pain was managed by the nurse and doctor team according to an algorithm based on the AHCPR guidelines.[31] The reduction in usual pain scores is shown in Figure 1. The standard group made no improvement, even when the practitioners knew the study was ongoing in their office. The intervention group had a significant reduction in pain scores, which was sustained.
TABLE 5

Measures of Care in Palliative Care Unit vs Other Hospital Units
• VCU Experience-In our institution, symptom evaluation by nursing staff and point-of-service corrections are important to the quality of care in our palliative care unit and translate into real patient benefits. As part of a quality improvement project (unpublished data on file), we recorded patient symptoms, treated according to an algorithm for each symptom, and remeasured symptoms scores as shown in Figure 2. Patient symptom scores were significantly improved, but of equal importance, the process of treating by algorithm created a standardized care-set with consistent care delivery despite provider differences. This ability to perform assessments, fix problems at the point of service, and avoid delays also has increased the satisfaction of the nurses on the unit, such that there has been less than 5% staff turnover in 5 years. Compared to the rest of VCU hospital, which shows results that are comparable to the other University Health-System Consortium hospitals audited, the VCU palliative care unit shows markedly improved scores in almost all categories (Table 5).
• Advance Directives-The issue of advance directives and the lack of progress in their use over the past 20 years[11] was mentioned above. A recent primary care-based project involved the mailing of advance directive forms to patients and prompted physicians to have such directives documented.[66] The prompts by themselves had no effect, but the mailed advance directives in addition to the prompts increased discussions between physicians and patients as well as the number of completed advance directives. To our knowledge, this has not been attempted in oncology practices.
• Economic Incentives-Other suggestions for potential projects to improve symptom management include economic incentives for documentation of symptom assessment and implementation of treatment strategies. The use of economic incentives, while reported to be somewhat successful in improving delivery of preventive medical care,[67] has not been reported in the symptom management literature. Another potential project would be to periodically audit five to seven random charts of practicing oncologists to assess for recognition, documentation, and treatment of symptoms, and then to provide each clinician with a "report card," complete with comparison to the (blinded) scores of their peers. Although this strategy has not been reported in the oncology literature, it may provide a strong incentive to improve symptom management.
Several insurers are making plans to institute incentive programs for oncology practices aimed at documenting quality of care and symptom control, but none have been fully realized yet. Many of these programs are proprietary, and data are not available. Finally, computerized prompts appear to be a promising and practical method for collecting symptom data and triggering clinicians to initiate an intervention.
Making This Doable in Practice
If recognizing symptoms, making them manifest, and treating them was easy, or obvious, or highly rewarded, it would be widely practiced already. There is a wide chasm between the idealistic intent to provide the highest quality patient care and the reality of daily clinical demands on the physician. The solutions are relatively straightforward, even if not always tested in a randomized clinical trial, for the barriers to optimal patient care have as much to do with sociology and psychology as they do with medicine.
In situations where decisions have a direct economic impact, fiscal incentives and disincentives are commonly used to alter behavior, but changing behavior because it is "the right thing to do" requires an unobtrusive approach that demands as little as possible from the clinician. A simple law of economics-opportunity cost-eloquently explains this concept. The extra time a physician takes to perform a task represents a cost, as that time could be used to see other patients, dictate charts, or perform other necessary work. If the extra time is reimbursable, then the incentive to perform the task may equal or overcome the opportunity cost (making it "worth the physician's time"). However, if the task is not reimbursable, then it must be simplified so as to minimize the opportunity cost to the physician. To make this possible, the utilization of nursing staff, algorithms, computer prompts, and other strategies are employed.
Identify the Problem
Determine if symptom control is a problem by using a symptom assessment scale in the office. The scales are standardized and require little extra time to complete. As discussed, if pain, fatigue, and nausea are addressed, the physician work may be reimbursable; this addresses the idea of incentive. Since the assessment scale is standardized, it requires only a little extra effort on the clinician's part, and making it quick and easy to obtain reduces the opportunity cost to the clinician. Naturally, if all scores are good, then there is no need for action.
Make the Problem Visible
Use the symptom assessment scale to make the problems clear and apparent on a problem list. Once a problem or problems are identified and actively addressed, less additional effort is required by the clinician to perform a general assessment with each subsequent visit.
Make It Easy to Address the Problem
Predetermined algorithms or symptom control plans for each of the most common symptoms can be developed. This entails a single-time investment by a clinical group to determine appropriate treatment pathways such as dose escalation for pain medication, algorithms to treat agitation, nausea, and depression, and a variety of other common issues. The algorithms involve physicians and nurses in the treatment plan rather than the physician alone, negating the problem of treatment delay due to an inability to locate the physician when urgent treatment is required. Likewise, the opportunity cost on the physician's time is placed at a minimum since the tasks are shared among a variety of healthcare workers.
We are happy to share our current algorithm list; those interested should contact program coordinator Carrie Cybulski (ccybulski@mail1.vcu.edu). Programs and practitioners that are interested in further integrating palliative care into oncology practice are urged to contact the Center to Advance Palliative Care (www.capc.org) and visit one of the Palliative Care Leadership Centers.
Financial Disclosure:L. Lyckholm, P. Coyne, and T. Smith all receive salary and research support by a grant from the Robert Wood Johnson Center to Advance Palliative Care (www.capc.org). T. Smith and J. Roberts receive research support from the National Cancer Institute. T. Smith receives unrestricted research support from Medtronic, Inc. T. Smith and P. Coyne have both received honoraria for speaking about trials supported by Medtronic, Inc.
References:
1. Foley KM: The treatment of cancer pain. N Engl J Med 313:84-95, 1985. 2. Von Roenn JH, Cleeland CS, Gonin R, et al: Physician attitudes and practice in cancer pain management. A survey from the Eastern Cooperative Oncology Group. Ann Intern Med 119:121-126, 1993.
3. Cleeland CS, Gonin R, Hatfield AK, et al: Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 330:592-596, 1994.
4. Hewitt ME, Simone JV (eds): Ensuring Quality Cancer Care. Washington, DC, National Academies Press, 1999.
5. Hillner BE, Smith TJ, Desch CE: Hospital and physician volume or specialization and outcomes in cancer treatment: Importance in quality of cancer care. J Clin Oncol 18:2327- 2340, 2000.
6. Grilli R, Minozzi S, Tinazzi A, et al: Do specialists do it better? The impact of specialization on the processes and outcomes of care for cancer patients. Ann Oncol 9:365-374, 1998.
7. Foley KM, Gelband H (eds): Improving Palliative Care for Cancer, pp 1-344. Washington, DC, National Academies Press, 2001.
8. National Cancer Policy Board: Improving Palliative Care: We Can Take Better Care of People With Cancer, pp 1-20. Washington, DC, National Academies Press, 2003.
9. Mertens W, Higby DJ, Brown D, et al: Improving the care of patients with regard to chemotherapy-induced nausea and emesis: The effects of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol 21:1373-1378, 2003.
10. Cohen L, De Moor C, Eisenberg P, et al: Delayed chemotherapy-induced nausea and vomiting (CINV) remains a problem and significantly interferes with daily function (DF) in pts receiving emetogenic chemotherapy (CT) in the United States (abstract 3973). Proc Am Soc Clin Oncol 22:739, 2003.
11. Teno JM: Advance directives: Time to move on. Ann Intern Med 141:159-160, 2004.
12. Emanuel EJ, Yinong Young-XU MA, Levinsky NG, et al: Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med 138:639-643, 2003.
13. Earle CC, Chapman R, Baker C, et al: Systematic overview of cost-utility assessments in oncology. J Clin Oncol 18:3302-3317, 2000.
14. Earle CC, Neville BA, Landrum MB, et al: Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 22:315-321, 2004.
15. Weeks JC, Cook EF, O’Day SJ, et al: Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA 279:1709-1714, 1998.
16. Smith TJ, Swisher K: Telling the truth about terminal cancer. JAMA 279:1746-1748, 1998.
17. Miaskowski C, Dodd MJ, West C, et al: Lack of adherence with the analgesic regimen: A significant barrier to effective cancer pain management. J Clin Oncol 19:4275-4279, 2001.
18. Desbiens NA, Wu AW: Pain and suffering in seriously ill hospitalized patients. J Am Geriatr Soc 48:S183-S186, 2000.
19. Demoor C, Cohen L, Eisenberg PD, et al: Oncologists’ compliance with antimetic guielines (AEG) and outcomes of patients (pts) receiving emetogenic chemotherapy (CT) (abstract 2924). Proc Am Soc Clin Oncol 22:727, 2003.
20. SUPPORT Principal Investigators: A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA 274:1591-1598, 1995.
21. Teno JM, Clarridge BR, Casey V, et al: Family perspectives on end-of-life care at the last place of care. JAMA 291:88-93, 2004.
22. Lutz S, Spence C, Chow E, et al: Survey on use of palliative radiotherapy in hospice care. J Clin Oncol 22:3581-3586, 2004.
23. van den Hout WB, van der Linden YM, Steenland E, et al: Single-versus multiple-fraction radiotherapy in patients with painful bone metastases: Cost-utility analysis based on a randomized trial. J Natl Cancer Inst 95:222- 229, 2003.
24. Billings JA, Gardner M, Putnam AT: A one-day, hospital-wide survey of dying inpatients. J Palliat Med 5:363-374, 2002.
25. Runciman WB, Merry AF, Tito F: Error, blame, and the law in health care-an antipodean perspective. Ann Intern Med 138:974- 979, 2003.
26. Tu K, Davis D: Can we lter physician behavior by educational methods? Lessons learned from studies of the management and follow-up of hypertension. J Cont Educ Health Prof 22:11-22, 2002.
27. Yabroff KR, Mandelblatt JS, Ingham J: The quality of medical care at the end-of-life in the USA: Existing barriers and examples of process and outcome measures. Palliat Med 18:202-216, 2004.
28. Cowan JD, Walsh D, Homsi J: Palliative medicine in a United States cancer center: A prospectve study. Am J Hosp Palliat Care 19:240-250, 2002.
29. Schrag D: The price tag on progress- chemotherapy for colorectal cancer. N Engl J Med 351:317-319, 2004.
30. Du Pen S, Du Pen A, Polossar N, et al: Implementing guidelines for cancer pain management: Results of a randomized controlled clinical trial. J Clin Oncol 17:361-370, 1999.
31. Jacox A, Carr DB, Payne R, et al: Management of Cancer Pain Clinical Practice Guideline No. 9 (AHCPR Publication No. 94- 0592). Rockville, Md, Agency for Health Care Policy and Research, US Department of Health and Human Services, Public Health Service, March 1994.
32. Smith TJ, Staats PS, Deer T, et al: Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J Clin Oncol 20:4040-4049, 2002.
33. Higginson IJ, Finlay I, Goodwin DM, et al: Do hospital-based palliative teams improve care for patients or families at the end of life? J Pain Symptom Manage 23:96-106, 2002.
34. Farrar JT, Young JP Jr, LaMoreaux L, et al: Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94:149-158, 2001.
35. McQuay HJ, Moore RA, Eccleston C, et al: Systematic review of outpatient services for chronic pain control. Health Technology Assess 1:1-135, 1997.
36. Miaskowski C, Dodd M, West C, et al: Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol 22:1713-1720, 2004.
37. Elsayem A, Swint K, Fisch MJ, et al: Palliative care inpatient service in a comprehensive cancer center: Clinical and financial outcomes. J Clin Oncol 22:2008-2014, 2004.
38. Smith TJ, Cassel JB, Coyne PJ, et al: Quality of care and cost savings associated with an inpatient high volume, standardized-care palliative care unit. J Palliat Med. In Press.
39. Allegre A: Building a palliative care program at your community cancer center. Oncology Issues May/June 2003.
40. Meier D: Planning a hospital-based palliative care program: A primer for institutional leaders. Available at www.capc.org.
41. Cuny J, et al: Palliative Care 2004 report. Available at www.uhc.edu.
42. Campbell DE, Lynn J, Louis TA, et al: Medicare program expenditures associated with hospice use. Ann Intern Med 140:269-277, 2004.
43. Naik G: Unlikely way to cut hospital costs: Comfort the dying. The Wall Street Journal. March 10, 2004.
44. Smith TJ, Girtman J, Riggins J: Why academic divisions of hematology/oncology are in trouble and some suggestions for resolution. J Clin Oncol 19:260-264, 2001.
45. Smith TJ, Desch CE, Hackney MH, et al: How long does it take to get a “do not resuscitate” order? J Palliat Care 13:5-8, 1997.
46. Sulmasy DP, Rahn M: I was sick and you came to visit me: Time spent at the bedsides of seriously ill patients with poor prognoses. Am J Med 111:385-389, 2001.
47. Bruera E, Kuehn N, Miller M, et al: The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 7:6-9, 1991.
48. Portenoy RK: Cancer pain: From curriculum to practice change. J Clin Oncol 10:1830-1832, 1992.
49. deHaes JCJM, Olschewski M, Fayers P, et al: Measuring the qualityof life of cancer patients with the Rotterdam Symptom Checklist (RSCL). A manual. Northern Centre for Healthcare Research, NCH series 9, Groningen, The Netherlands, 1996.
50. Guidelines for using the Edmonton Symptom Assessment Scale (ESAS). Available at www.palliative.org/PC/ClinicalInfo/ AssessmentTools/esas.pdf. Accessed July 26, 2005.
51. Davis DA, Thomson MA, Oxman AD, et al: Evidence for the effectiveness of CME: A review of 50 randomized controlled trials. JAMA 268:1111-1117, 1992.
52. Soumerai SB, Avorn J: Principles of educational outreach (“academic detailing”) to improve clinical decision making. JAMA 263:549-556, 1990.
53. Field MJ, Lohr KN (eds): Clinical Practice Guidelines. Washington, DC, Institute of Medicine, National Academies Press, 1990.
54. American Society of Clinical Oncology: Recommendations for the use of hematopoietic colony-stimulating factors: evidence based, clinical practice guidelines. J Clin Oncol 12:2471-2508, 1994.
55. Bennett CL, Weeks J, Somerfield MR, et al: Use of hematopoietic colony stimulating factors: Comparison of the 1994 and 1997 American Society of Clinical Oncology surveys regarding ASCO clinical practice guidelines. J Clin Oncol 17:3676-3681, 1999.
56. Grol R: Beliefs and evidence in changing clinical practice. BMJ 315:418-421, 1997.
57. Browman GP, Levine MN, Mohide EA, et al: The practice guidelines development cycle: A conceptual tool for practice guidelines development and implementation. J Clin Oncol 13:502-512, 1995.
58. Grimshaw JM: Effect of clinical guidelines on medical practice: A systematic review of rigorous evaluations. Lancet 342:1317-1322, 1993.
59. Smith TJ, Hillner BE: Ensuring quality cancer care by the use of clinical practice guidelines and critical pathways. J Clin Oncol 19:2886-2897, 2001.
60. Shea S, DuMouchel W, Bahamonde L: A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inform Assoc 3:399- 409, 1996.
61. Agrawal A, Mayo-Smith MF: Adherence to computerized clinical reminders in a large healthcare delivery network. Medinfo 11:111- 114, 2004.
62. Dexter PR, Perkins S, Overhage JM, et al: A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med 345:965-970, 2002.
63. Kralj B, Iverson D, Hotz K, et al: The impact of computerized clinical remnders on physician prescribing behavior: Evidence from community oncology practice. Am J Med Qual 18:197-203, 2003.
64. Khatcheressian J, Smith TJ, Lyckholm L, et al: Erythropoietin use in medically underserved patients (pts) (abstract 8139). Proc Am Soc Clin Oncol 23:759, 2004.
65. Roumie CL, Grogan EL, Falbe W, et al: A three-part intervention to change the use of hormone replacement therapy in response to new evidence. Ann Intern Med 141:118-125, 2004.
66. Heiman H, Bates DW, Fairchild D, et al: Improving completion of advance directives in the primary care setting: A randomized controlled trial. Am J Med 117:318-324, 2004.
67. Town R, Kane R, Johnson P, et al: Economic incentives and physicians’ delivery of preventive care: A systematic review. Am J Prev Med 28:234-240, 2005.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.