Improving Quality of Life, Not Transfusion Avoidance, Drives Clinical Use of Erythropoietin
DURHAM, North Carolina-Improving quality of life for cancer patients is the driving force behind clinical patterns of use of erythropoietin (EPO) therapy, at least in this country, according to Jeffrey Crawford, MD. Although the Food and Drug Administration approved epoetin alfa (Epogen, Procrit) based on evidence that it reduced the need for transfusions in cancer patients with chemotherapy-related anemia, most current clinical use of epoetin alfa is not to decrease transfusion needs. "I think we’re convinced now that there is a quality-of-life benefit," Dr. Crawford said (Figure 1), and epoetin alfa is now primarily directed at helping cancer patients realize that benefit
DURHAM, North CarolinaImproving quality of life for cancer patients is the driving force behind clinical patterns of use of erythropoietin (EPO) therapy, at least in this country, according to Jeffrey Crawford, MD. Although the Food and Drug Administration approved epoetin alfa (Epogen, Procrit) based on evidence that it reduced the need for transfusions in cancer patients with chemotherapy-related anemia, most current clinical use of epoetin alfa is not to decrease transfusion needs. "I think we’re convinced now that there is a quality-of-life benefit," Dr. Crawford said (Figure 1), and epoetin alfa is now primarily directed at helping cancer patients realize that benefit.
Dr. Crawford is professor of medicine in the divisions of oncology and hematology at Duke University in Durham, North Carolina, and director of clinical research at the Comprehensive Cancer Center at Duke University Medical Center.
Community-Based Trials
Three community-based studiesthe Glaspy, Demetri, and Gabrilove trialshave consistently demonstrated the relationship between improving hemoglobin levels and overall quality of life in cancer patients receiving chemotherapy. Although the trials were not randomized and had no control arms, the large number of patientsmore than 7,000 totalmake the database very robust, Dr. Crawford said. Together these studies represent one of the largest prospective, open-label, nonrandomized databases of any therapeutic agent in cancer research.
The studies all used linear analogue self-assessment (LASA) scores to measure quality of life (Table 1) and the Demetri and Gabrilove trials also included the Functional Assessment of Cancer Therapy-Anemia (FACT-An).
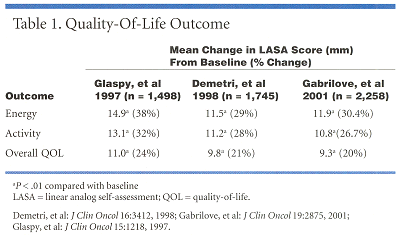
Across the trials, hemoglobin improved by 1.8 to 2.0 g/dL. The improvement in levels was not only associated with a significant reduction in the need for transfusions. The trials also "demonstrated the profound relationship between hemoglobin level and quality of life," Dr. Crawford stated. Quality of life measurements improved in a stepwise fashion and in direct relation to increases in hemoglobin levels, and were particularly significant among patients who achieved an improvement of 2 g/dL in hemoglobin levels.
Target Hemoglobin Level?
A separate analysis showed that the greatest increment of quality-of-life improvement occurred at a hemoglobin level of 12 g/dL, suggesting that this might be an important target level for cancer patients receiving chemotherapy. Work by Finch in 1971 showed a relationship between endogenous EPO and hemoglobin, with an inflection point at 12 g/dL. At or above a hemoglobin of 12, endogenous EPO is quite low, "but once you hit that point of 12, there is a dramatic rise in endogenous erythropoietin," Dr. Crawford said. "We’ve known that for over 30 years, but clinically we haven’t acted upon it until recently because we weren’t sure how that related to the clinical environment."
Careful analysis in the Demetri trials showed a consistent relationship between an increase in hemoglobin levels and improvement in energy, activity, and overall quality of life in patients with complete response, partial response, and stable disease. Patients with progressive disease, however, did not show an improvement in quality of life despite an increase in hemoglobin. "Both hemoglobin response and tumor response are important variables in patient outcome," Dr. Crawford stated.
The overall response data show 49% of patients in the Gabrilove study and 47% in the Demetri study responded initially. Increasing doses were required by about one-third of patients and pushed response rates to 68% in the Demetri study and 65% in the Gabrilove study, Dr. Crawford reported. These and other data can serve as benchmarks for the new data on darbepoetin alfa (Aranesp).
Results Confirmed
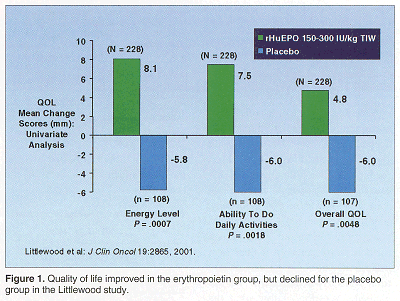
The Littlewood study, performed in Europe among patients receiving nonplatinum-based chemotherapy for hematologic cancers and a variety of solid tumors, randomized patients to EPO or placebo. Patients treated with EPO had an average improvement in hemoglobin of 2.2 g/dL compared to 0.5 g/dL for patients on placebo.
"Despite maintaining the hemoglobin level in the placebo group, the quality of life is actually decreasingpresumably either from the morbidity of the disease or its treatmentwhile the quality of life is improving in the EPO group" Dr. Crawford observed. "So you can’t take a static hemoglobin and relate it to quality of life. It’s a very dynamic process,
particularly over time."
"The trials of erythropoietic agents of the future needed to focus on higher levels of hemoglobin," Dr. Crawford said, to determine target levels of hemoglobin that will be beneficial to most patients as well as cost-effective. "In addition," he concluded, "we need a much better understanding of what maintenance of normal hemoglobin is going to mean for our patients, so we can move from a strategy not of treatment of anemia, but really of prevention."