Indications for Selective Neck Dissection: When, How, and Why
Selective neck dissection is a procedure that is primarily indicated in patients with clinically negative nodal disease in which there is a high risk of occult metastases. Others have advocated its use for patients with
ABSTRACT: Selective neck dissection is a procedure that is primarily indicated in patients with clinically negative nodal disease in which there is a high risk of occult metastases. Others have advocated its use for patients with positive nodes, although under very specific circumstances and in combination with postoperative radiation therapy. The type of selective neck dissection performed varies according to the site of the primary, because the pattern of metastases is unique in each case. This review presents the author’s philosophy on when, how, and why to employ the procedure, based on the location of primary cancers at oral, pharyngeal, laryngeal, cutaneous, thyroid, and salivary gland sites. [ONCOLOGY 14(10):1455-1464, 2000]
Introduction
TABLE 1
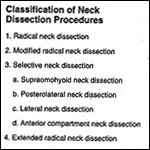
Classification of Neck Dissection Procedures TABLE 2
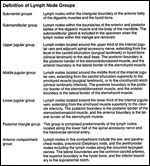
Definition of Lymph Node Groups FIGURE 1
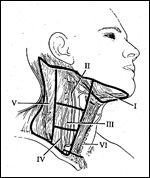
Cervical Lymph Nodes
Selective neck dissection is an operative procedure designed to remove cervical lymph nodes at risk for involvement by metastatic disease and is characterized by the preservation of one or more lymph node groups that are routinely removed in radical neck dissections.[1] According to the Committee for Head and Neck Surgery and Oncology of the American Academy of Otolaryngology/Head and Neck Surgery, selective neck dissection is one of four neck dissection procedures, and it includes four specific subtypes (Table 1). This committee also recommended terminology and defined boundaries to standardize the description of lymph node groups typically removed during neck dissection procedures (Table 2). The submental and submandibular lymph node groups are contained within level I. Levels II, III, and IV, respectively, include the superior, middle, and inferior jugular groups. Level V defines the posterior triangle nodes, and level VI contains the anterior compartment group (Figure 1).
Selective neck dissections are typically performed for occult or early metastases for which the removal of nonlymphatic structures (eg, sternocleidomastoid muscle, internal jugular vein, spinal accessory nerve) is thought not to be necessary. The procedure consists of compartmental removal of one or more levels containing lymph node groups determined to be at risk for metastatic cancer. Assessment of this risk is based on the size and location of the primary tumor. Thus, the compartments removed depend on the location of the primary lesion and its known pattern of spread.
Historical Perspective
The origin of the concept upon which selective neck dissection is based is unclear. Head and neck surgeons likely used this approach for several decades without describing the technique in a formal manner. For example, at the end of the 19th century, Kocher used limited neck surgery when resecting cancer of the oral cavity without clinically evident neck nodes.[2] Later, a limited procedure known as suprahyoid neck dissection became popular as a means of removing occult lymphadenopathy associated with cancers of the oral cavity, particularly cancer of the lip.
Following Lindberg’s paper describing skip metastases that develop in level II and III nodes without affecting level I, suprahyoid neck dissection fell into disfavor and was replaced by supraomohyoid neck dissection.[3] Suarez[4] and Bocca et al[5] described a modification of radical neck dissection for patients with laryngeal and hypopharyngeal cancers and clinically negative nodal disease. The procedure attracted much attention because it preserved the spinal accessory nerve, internal jugular vein, and sternocleidomastoid muscle. It became known as functional neck dissection, with the major emphasis being preservation of function.
Among the first to report the efficacy of limited neck surgery in a large series of patients, Jesse et al[6] independently developed neck dissection modifications for cancers of the oral cavity and pharynx. They referred to the procedures collectively as modified neck dissections, in which there was also an emphasis on preserving level I neck nodes for pharyngeal cancers and level V nodes for cancers of the oral cavity.
Later, Byers promoted their work further and used the terms “anterior” and “supraomohyoid” neck dissection to describe the selective neck dissection procedures performed for cancers of the oral cavity and pharynx.[7] The term “selective neck dissection” was not adopted to describe all the procedures encompassed within the sphere of limited dissection of the regional lymphatics until 1991.[1,8,9]
Rationale
The general rationale for using selective neck dissections is based on the topographic distribution of lymph node metastases. This distribution appears to be predictable in patients with previously untreated squamous cell carcinoma of the head and neck, particularly in early disease. Indeed, anatomic studies by Rouviere[10] and Fisch and Sigel[11] demonstrated that lymphatic drainage of the mucosal surfaces of the head and neck follows relatively constant and predictable routes.
In 1972, a clinical study by Lindberg[3] demonstrated that the lymph node groups most frequently involved in patients with carcinoma of the oral cavity are the jugulodigastric and midjugular nodes (levels II and III). In patients with carcinoma of the floor of the mouth, anterior oral tongue, and buccal mucosa, the nodes most frequently involved are in the submandibular triangle (level I). Lindberg also noted that cancers frequently metastasize to both sides of the neck and can skip the submandibular and jugulodigastric nodes, metastasizing first to the midjugular region.
The Lindberg study demonstrated that in the absence of metastases to the first echelon nodes, tumors of the oral cavity and oropharynx rarely involve the inferior jugular and posterior triangle nodes. Similar findings were reported by Skolnik et al in 1976,[12] with a study of radical neck dissection specimens that found no metastases in the nodes of the posterior triangle of the neck, regardless of the site of the primary tumor, or the presence or absence of metastases in the jugular nodes.
Further evidence supporting the concept that lymph node metastases follow predictable patterns of spread was provided by Shah[13] in a retrospective study of radical neck dissection specimens taken from patients with metastases from cancers of the oral cavity, larynx, and laryngopharynx. They concluded that cancers of the oral cavity metastasize most frequently to neck nodes in levels I, II, and III, whereas cancers of the oropharynx, hypopharynx, and larynx metastasize most frequently to the nodes in levels II, III, and IV.
General Indications
As a general rule, selective neck dissection is performed in patients with cancer arising in the head and neck region who are considered at risk for metastatic disease in the regional cervical lymph nodes. The procedure is indicated primarily in patients who have no evidence of clinical metastases, who have a 15% to 20% risk of harboring occult metastatic disease, and for whom surgery is the preferred treatment of the primary lesion.
Additional indications include situations in which surgical access to the primary cancer extends to lymph node groups at risk for metastases and, more controversially, clinical evidence of nodal metastases confined to the first echelon nodes (usually N1 disease) when the primary is to be treated by surgical removal. In this setting, the patient will most likely receive postoperative radiation therapy, and the purpose of the selective neck dissection is to eradicate all gross disease.
Previous reports on selective neck dissection have typically approached the subject based on the specific type of neck dissection performed; eg, supraomohyoid vs lateral. For this article, I have chosen to review the issues based on the site of origin of the cancer. With this approach, an analysis can be made that is applicable to patients presenting with the full spectrum of the disease. Within each of the sites in the head and neck, the indications for selective neck dissection vary. Furthermore, there are important nuances that influence the strategic approach.
Cancer of the Oral Cavity
When
Selective neck dissection plays an important role in the management of oral cavity cancer. The two most common subsites involved by squamous cell carcinoma arising in this region are the mobile (oral) tongue and the floor of the mouth. With the exception of very superficial involvement, mucosal lesions arising from each of these structures, including those found in patients with early disease (T1-2), have a high propensity to metastasize to the regional lymph nodes.[14]
The risk for lymph node metastases associated with carcinomas arising from other oral cavity subsites, such as the buccal mucosa, hard palate, lip, alveolar ridge, and retromolar trigone, appears to be more directly correlated to the size and extent of the primary lesion. Thus, for these latter subsites, selective neck dissection is recommended for the ipsilateral node-negative neck in patients with intermediate and advanced mucosal tumors.[15]
FIGURE 2A
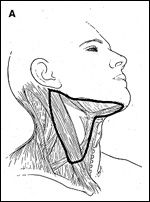
Selective Neck Dissection
The regional lymph nodes at greatest risk for involvement in patients with carcinoma of the oral cavity are located in levels I to III. The selective neck dissection designed to encompass these levels is often referred to as the supraomohyoid neck dissection-a procedure designed to remove the submental, submandibular, superior deep jugular (jugulodigastric), and middle deep jugular lymph node groups (Figure 2A).
For cancer of the lower lip, several studies support the use of suprahyoid neck dissection, in which only nodes in level I are removed.[16,17] However, suprahyoid neck dissection has essentially been abandoned for other cancers of the oral cavity, based on Lindberg’s report of skip metastases found in level II and III nodes and studies revealing unacceptable recurrence rates.[18-20] The morbidity accompanying the addition of level II and III nodes to the suprahyoid neck dissection procedure is primarily related to possible injury of the spinal accessory nerve, but the risk appears warranted for all oral cavity cancers including those of the lip. That said, early cancer of the lip (T1-2) has a low incidence of nodal spread; thus, elective neck dissection is not indicated.
Bilateral selective neck dissections are indicated for patients with primary lesions at risk for lymph node metastases involving the floor of the mouth, ventral surface, or midline tongue, and in whom there is no definite indication for postoperative radiotherapy.
There are also data to suggest that certain patients with node-positive disease can be treated effectively with selective neck dissection, provided postoperative radiation therapy is administered.[7,21-24] Such patients include those with a single palpable node located in the superior aspect of the neck (levels I to II). In this case, removal of levels I through IV is more appropriate.
How
A detailed description of the various techniques for selective neck dissection is beyond the scope of this review, and the reader is referred to other sources.[25] However, certain caveats will be highlighted as they relate to various clinical presentations.
Since the lymphatics draining the oral cavity, pharynx, and larynx do not involve the superficial pathways (eg, external jugular lymph nodes), it is not necessary to remove the fascia and soft-tissue structures lateral to the sternocleidomastoid muscle. The external jugular vein and the sensory branches of the greater auricular nerve can be preserved.
The spinal accessory nerve is dissected free of its surrounding fibrofatty tissue from the level of the skull base adjacent to the internal jugular vein, to its point of entry into the sternocleidomastoid muscle. It is necessary to dissect along the inferior border of the posterior belly of the digastric muscle and retract it superiorly to provide adequate exposure of the upper carotid sheath near the skull base. This triangle formed by the posterior belly of the digastric muscle, the spinal accessory nerve, and the sternocleidomastoid muscle outlines an area of tissue bearing lymph nodes subcategorized as level IIb (also referred to as the submuscular recess).[8]
The removal of these lymph nodes in patients with cancer of the oral cavity is controversial because studies have shown that disease in this region is only associated with the presence of metastatic disease in the area of level II lying anterior (medial) to the spinal accessory (referred to as level IIa).[26,27] The sensory branches of the cervical plexus can also be preserved by limiting the dissection laterally to a vertical plane corresponding to the posterior border of the sternocleidomastoid muscle.
All lymph nodes from level I can be completely removed by dissecting along the fascial planes of the muscles within the submental and submandibular triangle. This includes dissecting the preglandular nodes under the anterior belly of the digastric muscle and the pre- and postvascular nodes along the lower border of the body of the mandible. It is usually not necessary to remove the nodes lying along the facial artery as it courses lateral to the mandibular body, unless the primary cancer involves the buccal mucosa, maxillary alveolus, or upper lip.
Dissection of this latter nodal group (perifacial, buccinator) increases the risk of injury to the mandibular branch of the facial nerve. Metastases to the submental lymph nodes located within the submental triangle (level Ia) are most likely associated with cancer of the lower lip, floor of the mouth, anterior oral tongue, and buccal mucosa.[3] Thus, consideration should be given to preserving this subzone when performing selective neck dissection for other oral cavity sites, unless there is clinical evidence of metastases.
In cancer of the oral tongue, some evidence suggests that skip metastases can involve the inferior deep jugular lymph nodes (level IV).[28] Based on this observation, it is recommended that level IV be included along with levels I through III whenever a selective neck dissection is being performed for cancer of the oral tongue with an associated clinically negative neck.
Even when there is no clinical evidence of nodal disease, there is at least a 20% risk that occult disease is present in patients with these oral cavity lesions. Unless the treatment of choice for the primary lesion is radiotherapy, elective neck dissection should include removal of nodes in neck levels I through III (plus level IV for cancer of the tongue).
During the procedure, lymph nodes positive for metastatic disease may be encountered unexpectedly. If these nodes are confined to levels I and II, my preference is to continue with the selective neck dissection and remove levels I through IV. If the positive nodes involve levels III and IV, level V is also dissected. Postoperative radiation therapy should be administered in all such cases.
Some authorities believe that selective neck dissection is, in essence, a procedure for staging the neck in patients whose tumors are likely to produce metastases to the cervical lymph nodes.[29] Thus, among patients who undergo this procedure in conjunction with excision of the primary tumor, further information about the status of their nodal disease is revealed. In this way, patients who are found to have more aggressive tumors (and who are therefore at risk for recurrence in the neck) can be scheduled for more intensive therapy. For example, if there is pathologic evidence of lymph node metastases in the neck dissection specimen, then postoperative radiotherapy would be indicated.
Why
The data supporting the therapeutic efficacy of selective neck dissection for cancer of the oral cavity are primarily derived from retrospective analyses. The largest series includes patients treated at M. D. Anderson Cancer Center. The recurrence rate among 154 patients with pathologic node-negative disease (mostly cancer of the oral cavity) who had been treated with supraomohyoid neck dissection was 5.8%.[7] Several other reports have claimed similar results.[22-24,30]
An analysis of the results of selective neck dissection for node-positive disease shows more variability. For supraomohyoid dissections, Byers reported (in 1985) a regional failure rate of 15% among 80 patients with pathologically positive nodal disease.[7] Of these patients, 62 (78%) had multiple positive nodes, and 49 (61%) received postoperative radiotherapy. A 1997 report by Pellitteri and Robbins noted a regional recurrence rate of 11.1% in 27 patients with pathologically node-positive disease.[24]
In a 1999 analysis, Byers et al found that the regional recurrence rate for supraomohyoid neck dissection was 35.7% among patients with pathologic N1 disease who had not received radiation therapy, and 5.6% among those who did receive postoperative radiation therapy.[31] For pathologic N2b disease, the failure rate was 8.8% with postoperative radiation, and 14% without.
Ambrosch et al reported a recurrence rate of 6.6% (6 of 90 patients) among patients found to have pathologically node-positive disease involving both the oral cavity and pharynx.[22] Traynor et al reported a neck recurrence in only 1 of 29 patients who underwent 36 selective neck dissections (17 supraomohyoid, 19 lateral) for clinically and pathologically node-positive disease.[21] Spiro et al reported a neck recurrence in 2 (6%) of 31 patients with clinically and pathologically positive nodal disease undergoing supraomohyoid neck dissection.[23] Most patients in these series received postoperative radiation therapy.
Cancers of the Pharynx and Larynx
When
Cancers arising in the pharyngeal structures (nasopharynx, oropharynx, hypopharynx) and the larynx commonly metastasize to the regional lymph nodes. Lymphatic drainage of these locations is frequently bilateral, although this varies among the various subsites. Selective neck dissection has no role in the management of nasopharyngeal cancer, because radiation therapy (with or without chemotherapy) is the treatment of choice for disease at this primary site as well as the lymph nodes. Radical or modified radical neck dissection is recommended for persistent or recurrent lymphadenopathy.[32]
In oropharyngeal cancer (ie, involving the tonsil, base of tongue, or oropharyngeal wall), selective neck dissection is indicated only in certain circumstances. Early oropharyngeal cancer (T1-2) is best treated with radiation therapy, including both sides of the neck in the field. However, if there is clinical evidence of nodal disease, neck dissection may have a role in treatment.
Neck dissection is recommended for patients whose necks are staged N2 or greater, regardless of their response to radiation therapy, and salvage neck dissection is recommended for patients with persistent or recurrent adenopathy.[33] Although controversial, radical or modified radical neck dissection is indicated in either situation, particularly when there are no data to support the use of selective neck dissection.
Selective neck dissection is indicated for cancer of the oropharynx when the primary tumor is initially treated by surgery and there is no evidence of clinical nodal disease. If postoperative radiation therapy is indicated, it is unnecessary to perform a bilateral selective neck dissection because radiation alone is effective in treating the node-negative contralateral neck. However, if there is clinically positive contralateral disease, a bilateral neck dissection is indicated. Preferably, the procedure should remove all five levels of the neck on the side that contains the clinical disease.
Cancers arising in the hypopharynx frequently metastasize, with both sides of the neck at risk for involvement. Bilateral selective neck dissection is recommended for most patients with clinically node-negative disease. In supraglottic and advanced glottic cancer, there is greater controversy over whether or not to dissect the node-negative neck. This is particularly relevant if the primary lesion is to be treated surgically without transgressing the node-bearing tissue, as in endoscopic laser resection. There are data to support both elective neck dissection[22] and observation[34] in these patients. Neck dissection is not indicated for early glottic lesions, because they have a low rate of nodal metastases.[35]
Tumors confined to the lateral wall of the piriform sinus[36] and glottis[37] typically do not metastasize to the contralateral nodes. Nevertheless, I prefer to perform a bilateral neck dissection for all patients undergoing laryngectomy, because there is overlap between the surgical field and the node-bearing tissue on both sides of the neck. Even when postoperative radiation therapy is planned, it is advantageous to remove nodes on both sides of the neck when the nodal tissue is at risk for metastases. This eliminates the disadvantage of treating occult disease in close proximity to a surgical field with radiation therapy alone.
How
FIGURE 2B
Selective Neck Dissection
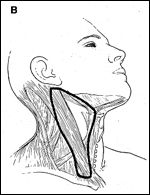
For cancer of the oropharynx, the levels of the neck at greatest risk for involvement are II, III, and IV. The selective neck dissection designed to remove these levels is often referred to as lateral neck dissection (Figure 2B).[1] Cancer of the oropharynx has a propensity to metastasize to both sides of the neck.[5] Therefore, unless postoperative radiation therapy is part of the treatment regimen, both sides of the neck should be dissected. The lymphatic drainage from the oropharynx includes the upper jugular nodes (level II) and the retropharyngeal nodes located medial to the carotid sheath.[10] The latter should also be removed as part of the neck dissection.[38]
For cancers of the larynx and hypopharynx, removal of nodes in levels II to IV on each side of the neck is also indicated. As with cancer of the oral cavity, the decision to remove level IIb is controversial, since recent reports indicate that this region is not at risk of involvement in the absence of clinically evident metastases.[26,27] Others have reported that level IV is not at risk of involvement in the absence of clinically positive nodes.[22,34]
Ambrosch et al reported only 9 neck recurrences in 163 patients with oral and pharyngeal cancers who underwent an elective selective neck dissection for clinically node-negative disease.[22] Their policy was to dissect only levels II and III for patients with pharyngeal lesions, while level IV was removed only when positive nodes were suspected at the time of surgery. Postoperative radiation therapy was administered to 83 patients. Tu reported a failure rate in the neck of 10.6% (15/142) among patients with pathologically node-negative supraglottic cancer who underwent dissection of level II nodes only.[39]
Since these data are contrary to accepted concepts, further studies are needed to confirm the practice of omitting level IV for clinically node-negative patients with cancer of the pharynx. As with cancer of the oropharynx, tumor involvement of the pharyngeal wall warrants removal of the retropharyngeal lymph nodes.
It is important to distinguish between removing only the node-bearing tissue overlying the internal jugular vein (so-called “jugular stripping”) and completely removing nodes within the boundaries of levels III and IV. Spiro et al found a pattern of neck failure in six patients after jugular node dissection, suggesting incomplete removal of the nodes lying posterior to the vein.[40] Byers et al also found evidence of recurrence within this field, underscoring the importance of removing the lymphatic chain lying immediately posterior to the internal jugular vein.[28]
Cancers arising in the pharyngeal and laryngeal structures tend to metastasize to the nodes in the central compartment (level VI).[41] This scenario is more likely to occur with advanced cancer of the larynx and hypopharynx extending to or below the level of the glottis. In such cases, dissection of the paratracheal and perithyroidal nodes in this region is recommended.
A recent report points to the risk of level I involvement in patients with supraglottic cancer. Of the 17 patients with clinically node-negative but pathologically node-positive disease, 14 (82%) had involvement of level I and 100% had involvement of level II. Thus, selective neck dissection in patients with supraglottic cancer should include level I (particularly the submandibular lymph nodes, referred to as level Ib) whenever there is evidence of positive nodes in level II.[42]
Why
The evidence to support the use of selective neck dissection in pharyngeal and laryngeal cancers is based on the efficacy data and observations of metastatic patterns. With regard to patterns of spread, Candela et al found only one patient (0.3%) with evidence of skip metastases outside of levels II to IV for both oropharyngeal and hypopharyngeal primaries.[43] With laryngeal primaries, level V was rarely involved, but always in conjunction with nodal metastases in levels II to IV.[44] Level I involvement was also rare and was accompanied by involvement of levels II to IV 75% of the time.
Efficacy data are based on studies by Byers and others who have analyzed recurrence rates following lateral neck dissection. Byers reported a regional recurrence rate of 3.9% among 256 patients with pathologically node-negative disease, 126 of whom had received postoperative radiotherapy.[7] Pellitteri et al reported similar findings for clinically node-negative disease.[24]
Byers also reported a regional recurrence rate of 7.3% among 41 patients with pathologically node-positive disease who underwent lateral neck dissection (37 of whom had postoperative radiotherapy).[7] Pellitteri and colleagues found the regional recurrence rate for 21 patients with pathologically node-positive disease to be 4.8%.[24]
In patients with multiple positive nodes, Byers et al recently reported a regional failure rate of 30% for those treated with postoperative radiation and 33% for those who did not receive the adjuvant radiotherapy.[31] In this series, the site of disease failure occurred most commonly within the dissected field, suggesting that removal of additional levels may not have changed the outcome. Nevertheless, this rate of recurrence is much higher than previously reported and should be considered a warning against the use of selective neck dissection when there is evidence of multiple positive nodes.
There are also data supporting the use of selective neck dissection in patients with bulky nodal metastases (N2-3) associated with carcinoma of the upper aerodigestive tract. Byers et al reported the outcome for patients with a small mucosal primary and advanced nodal disease (mass > 3 cm) thought to be locally controllable with radiotherapy.[45] Treatment for these patients consisted of selective neck dissection with removal of the obvious neck metastases, followed by radiotherapy to the primary and regional lymph nodes. The first site of recurrence was the neck in only 4 (11%) of the 35 patients in this highly selected group.
Investigators are currently studying the applicability of selective neck dissection following chemoradiation in patients with bulky neck metastases associated with advanced (T3-4) mucosal lesions of the upper aerodigestive tract. At the University of Tennessee, we have shown that patients treated with targeted cisplatin (Platinol) chemotherapy and concomitant radiation therapy (RADPLAT) achieve excellent regional control following selective removal of lymph nodes in the levels of the neck considered to be at greatest risk. All patients received a definitive dose of radiation therapy (68 to 74 Gy) along with four cycles of high-dose intra-arterial cisplatin; the neck dissection was performed 2 months after radiation therapy.[46] The limited surgery enabled removal of residual adenopathy while minimizing neck fibrosis, a sequela of the chemoradiation effects on soft tissue.
Other Sites
Selective neck dissection is used for other head and neck tumors besides those arising in the upper aerodigestive tract.
Skin Cancer
There are far fewer data available to support the use of selective neck dissections with skin cancer. Therefore, I will attempt to summarize the policy based more on biological principles than on clinical evidence.
FIGURE 2C
Selective Neck Dissection
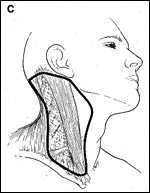
In managing skin cancers, it is important to know the propensity for nodal spread and the likely pathway. For cutaneous malignancies of the posterior upper neck and scalp, a posterolateral neck dissection is indicated for patients with clinically negative nodal disease who are undergoing resection of the primary lesion and whose regional lymph nodes are considered to be at risk (eg, malignant melanoma).[47] This procedure involves removal of neck nodes in levels II through V as well as the postauricular, suboccipital, and external jugular lymph node groups (Figure 2C). However, preliminary reports of sentinel node biopsy indicate that it may not be necessary to remove all of the nodes if there is no evidence of metastases within the sentinel nodes.[48,49]
Thyroid Cancer
FIGURE 2D
Selective Neck Dissection
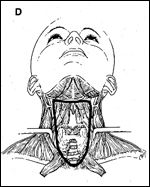
In papillary carcinoma of the thyroid, the anterior neck dissection is designed to eradicate nodal metastases associated with the primary lesion (Figure 2D). The procedure is indicated for large lesions that have extended through the capsule and for those in which there is evidence of lymphadenopathy within the central compartment.
For medullary carcinoma of the thyroid, the neck dissection should also encompass the jugular and posterior triangle lymph nodes.[50-52] For follicular carcinoma of the thyroid, neck dissection is performed only when there is evidence of positive nodal disease.
Anterior (central compartment) neck dissection involves removal of the perithyroidal nodes, pretracheal and paratracheal nodes along the cervical portion, precricoid (Delphian) nodes, and nodes located along each recurrent laryngeal nerve.[1] The boundaries of dissection include the carotid sheaths (laterally), the hyoid bone (superiorly), and the suprasternal notch (inferiorly).
Thorough dissection of the entire compartment will commonly disrupt the blood supply to the parathyroid glands, necessitating reimplantation. In most cases, this can be avoided by minimizing the dissection along the tracheoesophageal groove on the contralateral side in patients whose disease is confined to the ipsilateral gland.
The presence of nodal metastases as far inferiorly as the suprasternal notch indicates the possibility that the superior mediastinum is involved.[52] Optimal surgical exposure of this region requires either removing the manubrium and possibly one or both heads of the sternocleidomastoid muscle.[53] Some authors believe, however, that minimal disease in the superior mediastinum can be removed through the cervical approach alone.[54]
Salivary Gland Cancer
The value of selective neck dissection in cancer of the salivary gland is very controversial. The incidence of associated metastases to the regional nodes for high-grade malignant tumors arising from the parotid and submandibular glands is at least 20%. If there is clinically positive nodal disease, neck nodes in levels I through V should be treated by a therapeutic neck dissection. However, if the neck is clinically node-negative, selective neck dissection has been advocated as part of the surgical treatment.
For cancer of the parotid gland, levels Ib, IIa, IIb, and III would appear to be at greatest risk for involvement. In cancer of the submandibular gland, levels Ia, Ib, IIa, and III are most likely to have occult metastatic disease. Others have argued that patients with high-grade cancer of the salivary gland should receive postoperative radiation therapy that also effectively addresses the occult nodal disease.[55,56] Nonetheless, the oncologic principle of removing the node-bearing tissue at risk whenever it is located within the surgical field lends credence to performing the selective neck dissection at the time of the parotidectomy, particularly if part of the surgical exposure is through the upper neck.[55]
Ongoing Controversies
Although the selective neck dissection has become established as the technique of choice for surgeons treating cervical lymph node metastases, it is not without some degree of controversy.
Selective vs Modified Radical Neck Dissection
Should selective neck dissection be substituted for modified radical neck dissection in patients with clinically node-negative disease at high risk for occult metastases? Leemans et al argue that selective neck dissection is less effective, compared to the modified radical neck dissection.[57] They performed a meta-analysis comparing 688 patients treated with a selective neck dissection to 491 patients treated with modified radical neck dissection. The overall rate of recurrence in the ipsilateral neck was 3.3% vs 6.8%, respectively, for modified radical neck dissection vs selective neck dissection (P = .008).
However, there was no significant difference for pathologically node-negative patients, and it is unclear how many patients in the selective neck dissection group with pathologically node-positive disease had postoperative radiation therapy. If all patients with pathologically node-positive disease had received postoperative radiation therapy, as currently recommended, would the conclusion have been different?
Quality of Life
A discussion of selective neck dissection would not be complete without considering the functional outcome and its effects on quality of life. In particular, the frozen shoulder syndrome, which can develop following permanent injury to the spinal accessory nerve, can be a debilitating condition. Fortunately, the incidence of this complication has been markedly diminished through the use of techniques that preserve the motor innervation to the trapezius. Moreover, the risk for temporary nerve dysfunction appears to be significantly greater in patients undergoing modified radical neck dissection than in those treated with the selective procedures, and there is also a greater negative impact on quality of life.[58]
Conclusions
The strategy of selectively removing only lymph node groups at greatest risk for metastases in patients with head and neck cancers has evolved throughout the 20th century. Selective neck dissection has been used in the treatment of patients with cancer of the upper aerodigestive tract (oral cavity, pharynx, larynx), cancer of the thyroid gland, cutaneous malignancies, and cancer of the salivary gland. The type of selective neck dissection used varies with cancers at each major organ site, and there are many further variations of each technique. To avoid pitfalls and subsequently poor outcomes, a thorough knowledge of the nuances of each of these procedures for each subsite is important. Surgical technique and experience are also key factors for obtaining good results.
Most authorities accept the use of selective neck dissection for clinically node-negative disease, but its role in the management of clinically node-positive disease remains controversial. Nevertheless, data support its applicability in carefully selected circumstances.
The lower morbidity associated with selective neck dissection, relative to radical neck dissection and modified radical neck dissection, serves as an impetus to continue to study this strategy, to determine its efficacy in the management of head and neck cancer patients. This is relevant for patients with early nodal disease; combined with radiation therapy, its use in these patients may provide better functional outcomes and equal therapeutic efficacy, compared with more extensive neck surgery. The role of limited neck dissection after combined-modality therapy in patients with advanced disease also warrants further investigation.
References:
1. Robbins KT, Medina JE, Wolfe GT, et al: Standardizing neck dissection terminology. Arch Otolaryngol 117:601-605, 1991.
2. Kocher: Ueber Radical heilung des Krebses. Deutsche Ztschr F Chir 13:134-166, 1880.
3. Lindberg R: Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 29:1446-1449, 1972.
4. Suarez O: El de las metastasis problema linfaticas y alejadas del cancer de laringe e hipofaringe. Rev Otorrinolaryngol Santiago 23:83-99, 1963.
5. Bocca E, Pignataro O, Sasaki CT: Functional neck dissection: A description of operative technique. Arch Otolaryngol 106:524-527, 1980.
6. Jesse RH, Ballantyne AJ, Larson D: Radical or modified neck dissection: A therapeutic dilemma. Am J Surg 136:516-519, 1978.
7. Byers RM: Modified neck dissection: A study of 967 cases from 1970 to 1980. Am J Surg 150:414-421, 1985.
8. Suen JY, Goepfert H: Standardization of neck dissection nomenclature [editorial]. Head Neck Surg 10:75-77, 1987.
9. Medina JE: A rational classification of neck dissections. Otolaryngol Head Neck Surg 100(3):169-176, 1989.
10. Rouviere H: Anatomy of the Human Lymphatic System. Ann Arbor, Michigan, Edward Brothers, 1938.
11. Fisch UP, Sigel ME: Cervical lymphatic system as visualized by lymphography. Ann Otol Rhinol Laryngol 73:869-882, 1964.
12. Skolnik EM, King FY, Friedman M, et al: The posterolateral triangle in radical neck surgery. Arch Otolaryngol 102:1, 1976.
13. Shah JP: Patterns of lymph node metastases from squamous carcinomas of the upper aerodigestive tract. Am J Surg 160(4):405-409, 1990.
14. Lydiatt DD, Robbins KT, Byers RM, et al: Treatment of stage I and II oral tongue cancer. Head Neck 15(4):308-312, 1993.
15. Byers RM, Wolf PF, Ballantyne AJ: Rationale for elective modified neck dissection. Head Neck 10(3):160-167, 1988.
16. Koc C, Akyol MU, Celikkanat S, et al: Role of suprahyoid neck dissection in the treatment of squamous cell carcinoma of the lower lip. Ann Otol Rhinol Laryngol 106(9):787-789, 1997.
17. Cruse CW, Radocha RF: Squamous cell carcinoma of the lip. Plast Reconstr Surg 80(6):787-791, 1987.
18. Patterson HC, Dobie RA, Cummings CW: Treatment of the clinically negative neck in floor of the mouth carcinoma. Laryngoscope 94(6):820-824, 1984.
19. Donegan JO, Gluckman JL, Crissman JD: The role of suprahyoid neck dissection in the management of cancer of the tongue and floor of the mouth. Head Neck Surg 4(3):209-212, 1982.
20. Chu W, Strawitz JG: Results in suprahyoid, modified radical, and standard radical neck dissections for metastatic squamous cell carcinoma: Recurrence and survival. Am J Surg 136(4):512-515, 1978.
21. Traynor SJ, Cohen JI, Gray J, et al: Selective neck dissection and the management of the node-positive neck. Am J Surg 172(6):654-657, 1996.
22. Ambrosch P, Freudenbert L, Kron M, et al: Selective neck dissection in the management of squamous cell carcinoma of the upper digestive tract. Eur Arch Otorhinolaryngol 253(6):329-335, 1996.
23. Spiro RH, Morgan GJ, Strong EW, et al: Supraomohyoid neck dissection. Am J Surg 172(6):650-653, 1996.
24. Pellitteri PK, Robbins KT, Neuman T: Expanded application of selective neck dissection with regard to nodal status. Head Neck 19(4):260-265, 1997.
25. Robbins KT: Neck dissection, in Cummings CW, Harker LA, Krause CJ, et al (eds): Otolaryngology Head and Neck Surgery, 3rd ed. St Louis, Mosby, 1998.
26. Kraus DH, Rosenberg DB, Davidson BJ, et al: Supraspinal accessory lymph node metastases in supraomohyoid neck dissection. Am J Surg 172(6):646-649, 1996.
27. Talmi YP, Hoffman HT, Horowitz Z, et al: Patterns of metastases to the upper jugular lymph nodes (the “submuscular recess”). Head Neck 20(8):682-686, 1998.
28. Byers RM, Weber RS, Andrews T, et al: Frequency and therapeutic implications of “skip metastases” in the neck from squamous carcinoma in the oral tongue. Head Neck 19:14-19, 1997.
29. O’Brien CJ, Soong SJ, Urist MM, et al: Is modified radical neck dissection only a staging procedure? Cancer 59:994-999, 1987.
30. O’Brien CJ: A selective approach to neck dissection for mucosal squamous cell carcinoma. Aust N Z J Surg 64(4):236-241, 1994.
31. Byers RM, Clayman GL, McGill D, et al: Selective neck dissections for squamous carcinoma of the upper aerodigestive tract: Patterns of regional failure. Head Neck 21(6):499-505, 1999.
32. Wei WI, Ho CM, Wong MP, et al: Pathologic basis of surgery in the management of postradiotherapy cervical metastasis in nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 118(9):923-930, 1992.
33. Lavertu P, Adelstein DJ, Saxton JP, et al: Management of the neck in a randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer. Head Neck 19(7):559-566, 1997.
34. Gallo O, Boddi V, Bottai GV, et al: Treatment of the clinically negative neck in laryngeal cancer patients. Head Neck 18(6):566-572, 1996.
35. Yang CY, Andersen PE, Everts EC, et al: Nodal disease in purely glottic carcinoma: is elective neck treatment worthwhile? Laryngoscope 108(7):1006-1008, 1998.
36. Johnson JT, Bacon GW, Myers EN, et al: Medial vs lateral wall pyriform sinus carcinoma: Implications for management of regional lymphatics. Head Neck 16:401, 1994.
37. Hao SP, Myers EN, Johnson JT: T3 glottic carcinoma revisited: Transglottic vs pure glottic carcinoma. Arch Otolaryngol Head Neck Surg 121(2):166-170, 1995.
38. Ballantyne AJ: Significance of retropharyngeal nodes in cancer of the head and neck. Am J Surg 108:500, 1964.
39. Tu GY: Upper neck (level II) dissection for N0 neck supraglottic carcinoma. Laryngoscope 109(3):467-470, 1999.
40. Spiro RH, Gallo O, Shah JP: Selective jugular node dissection in patients with squamous carcinoma of the larynx or pharynx. Am J Surg 166(4):399-402, 1993.
41. Weber RS, Marvel J, Smith P, et al: Paratracheal lymph node dissection for carcinoma of the larynx, hypopharynx, and cervical esophagus. Otolaryngol Head Neck Surg 108(1):11-17, 1993.
42. Hicks WL Jr, Kollmorgen DR, Kuriakose MA, et al: Patterns of nodal metastasis and surgical management of the neck in supraglottic laryngeal carcinoma. Otolaryngol Head Neck Surg 121(1):57-61, 1999.
43. Candela FC, Kothari K, Shah JP: Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head Neck 12(3):197-203, 1990.
44. Candela FC, Shah J, Jaques DP, et al: Patterns of cervical node metastases from squamous carcinoma of the larynx. Arch Otolaryngol Head Neck Surg 116(4):432-435, 1990.
45. Byers RM, Clayman GL, Guillamondegui OM, et al: Resection of advanced cervical metastases prior to definitive radiotherapy for primary squamous carcinomas of the upper aerodigestive tract. Head Neck 14(2):133-138, 1992.
46. Robbins KT, Wong FS, Kumar P, et al: Efficacy of targeted chemoradiation and planned selective neck dissection to control bulky nodal disease in advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 125(6):670-675, 1999.
47. Rochlin DB: Posterolateral neck dissection for malignant neoplasms. Surg Gynecol Obstet 115:369-373, 1962.
48. O’Brien CJ, Uren RF, Thompson JF, et al: Prediction of potential metastatic sites in cutaneous head and neck melanoma using lymphoscintigraphy. Am J Surg 170(5):461-466, 1995.
49. Wagner JD, Park HM, Coleman JJ, et al: Cervical sentinel lymph node biopsy for melanomas of the head and neck and upper thorax. Arch Otolaryngol Head Neck Surg 126(3):313-321, 2000.
50. Ellenhorn JDL, Shah JP, Brennan MF: Impact of therapeutic regional lymph node dissection for medullary carcinoma of the thyroid. Surgery 114(6):1078-1082, 1993.
51. Fleming JB, Lee JE, Bouvet M, et al: Surgical strategy for the treatment of medullary thyroid carcinoma. Ann Surg 230(5):697-707, 1999.
52. Moley JF, DeBenedetti MK: Patterns of nodal metastases in palpable medullary thyroid carcinoma. Ann Surg 229(6):880-888, 1999.
53. Noguchi S, Murakami N: The value of lymph node dissection in patients with differentiated thyroid cancer. Surg Clin North Am 67(2):251-261, 1987.
54. Roka R, Niederle B, Fritsch A: Die transcervicale and transsternal dissektion des mediatinums beim carcinom der schilddruese. Langenbecks Arch Chir 359:5-7, 1983.
55. Kelley DJ, Spiro RH: Management of the neck in parotid carcinoma. Am J Surg 172(6):695-699, 1996.
56. Frankenthaler RA, Byers RM, Luna MA, et al: Predicting occult lymph node metastasis in parotid cancer. Arch Otolaryngol Head Neck Surg 119(5):517-520, 1993.
57. Leemans CR, Snow GB: Is selective neck dissection really as efficacious as modified radical neck dessection for elective treatment of the clinically negative neck in patients with squamous cell carcinoma of the upper respiratory and digestive tracts? Arch Otolaryngol Head Neck Surg 124(9):1042-1044, 1998.
58. Kuntz AL, Weymuller EA Jr: Impact of neck dissection on quality of life. Laryngoscope 109:1334-1338, 1999.