Influx of Funding Impels Collaborative Explorations to Study Sarcoma as Behavior Model for Other Cancers
This rare cancer finally sheds its FDA designation as an orphan disease.
ABSTRACT: This rare cancer finally sheds its FDA designation as an orphan disease.
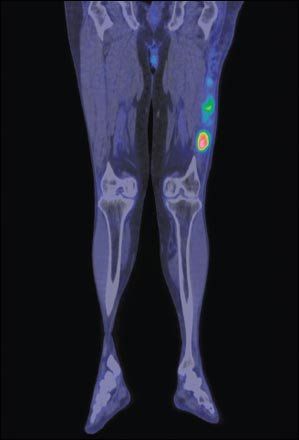
FDG-PET/CT fusion image of the legs, frontal section, showing liposarcoma of the left thigh. Liposarcoma is a malignant tumor that develops in deep soft fatty tissue.
Sarcoma accounts for just 1% of all cancers in the U.S. annually, so its designation by the FDA as an orphan disease is not surprising. Despite its rarity, these bone tumors have emerged as an important model for how more common cancers behave. In fact, drugs in development for sarcoma treatment are proving to be effective in ovarian, lung, and breast cancer. This orphan disease is finding a home in the cancer care continuum and experts told Oncology News International that there has never been a better time to be engaged in sarcoma research.
"The U.S. government has identified sarcoma as one of the diseases they are going to help, over the next several years, to find ways to treat and ways, hopefully, to cure," said Gary K. Schwartz, MD, chief of the Melanoma & Sarcoma Service at Memorial Sloan- Kettering Cancer Center (MSKCC) in New York. Dr. Schwartz's lab is currently working with grants from NCI and the American Recovery & Reinvestment Act (ARRA) to learn more about the molecular pathways of the disease.
"I would say there is a great deal of hope," said Lee Cranmer, MD, PhD. "Sarcomas are very tough nuts to crack, but we are making progress. Future generations-and I don't mean many generations, I mean the next generation-will not have the same burden of this problem as we experience now." Dr. Cranmer is an associate professor of clinical medicine at the Arizona Cancer Center, University of Arizona in Tucson.

GARY K. SCHWARTZ, MD
Oncology News International spoke with sarcoma specialists for an update on the current treatment options and what the future holds.
Genetic blueprint
Chemotherapy, radiotherapy, and surgery have been the primary treatment modes for sarcoma. Researchers have begun to look at the molecular biology of the different sarcoma subtypes in order to pin down targets that can be inhibited with drugs.
"That's the tack we've been taking for the last two or three years, to really dissect the sarcoma cell and begin to see the proteins and the pathways that are critical for those sarcoma cells to survive and find ways to block or interrupt those pathways with new small-molecule, targeted agents," said Dr. Schwartz, who is also a professor of medicine at Weill Cornell Medical College in New York.

LEE CRANMER, MD, PHD
With the NCI and ARRA funding, Dr. Schwartz and colleagues at the MSKCC Laboratory of New Drug Development are conducting studies on an antibody that blocks the insulin-like growth factor receptor (IGF-1R) that is commonly found in sarcoma cells. The antibody is being studied in combination with the mTOR inhibitor temserolimus (Torisel). "Other studies are being planned that will target the sonic hedgehog pathway and the notch pathway in patients with metastatic sarcoma," Dr. Schwartz said.
Researchers have begun to do gene arrays and DNA examinations to discover the actual genes unique to each sarcoma subtype. "We have to look at each subtype, and then at the specific genes, and then discover if there is a drug that can block those genes-and do this for every specific sarcoma subtype. That's the next step in refining sarcoma research, and those studies are just about to begin," Dr. Schwartz added.

JONATHAN TRENT, MD
About half of sarcomas are caused by specific, well-defined genetic events, such as a mutation or deletion or amplification, explained Jonathan Trent, MD, an associate professor at Houston's M.D. Anderson Cancer Center. "We are finding now that each different sarcoma is caused by a specific genetic event, and treatment of those specific tumors has to be individualized to the specific histology. This highlights the importance of getting a sarcoma patient's genetic blueprint."
"If there is one gene that is causing this cancer, surely we can develop therapies to target these genes. We can develop a therapy to target the gene that causes the cancer and then we stand a good chance of helping a lot of people. That is a very attractive prospect to me," he added.
Targeted agents
The oncogene KIT or CD117 is responsible for gastrointestinal stromal tumors and the targeted agent that has proven most effective in treating this subtype is imatinib (Gleevec). The drug has been useful in dermatofibrosarcoma, which has an amplification of the PDGF gene. When the latter is inhibited with imatinib, it becomes possible to shrink the tumor and convert patients from inoperable to operable.
Vascular endothelial growth factor (VEGF) receptor inhibitors such as sunitinib (Sutent) and sorafenib (Nexavar) have emerged as therapies for angiosarcoma and hemangioendothelioma. Response rates in patients with hemangiopericytoma who are resistant to standard chemotherapy are in the 70% range, Dr. Trent said.
Danosumab (Prolia), a RANK ligand inhibitor, is turning out to be active in patients with giant cell tumor of bone while the IGF-1R inhibitor R1507 shows promise in Ewing's sarcoma that has been heavily pretreated with chemotherapy.
"I think that sarcoma, although it is a rare entity, is one of the better, if not the best, model systems to study new targeted therapies because the 60 or so different types of sarcomas are caused by specific genetic events. Targeting those pathways has been proven to help patients with a number of different sarcomas," he added.
Collaborating to overcome challenges
TABLE Sarcoma subtypes
Because of sarcoma's rarity and multiple subtypes (see Table), it is difficult for any single institution to mount clinical trials. As a result, sarcoma researchers are joining forces both in the U.S. and internationally, most notably through the Sarcoma Alliance for Research through Collaboration (SARC), the World Sarcoma Network, and the Connective Tissue Oncology Society (CTOS).

ROBERT MAKI, MD, PHD
"SARC is a stand-alone group that coordinates centers to perform clinical trials together," said Robert Maki, MD, PhD, section chief for adult sarcoma oncology in the department of medicine at MSKCC. "It's led by Denise Reinke, MS, NP, and a board of scientific directors, and is an excellent example of a successful collaborative sarcoma clinical trials effort" (see Related Reading).
The European Organization for Research and Treatment of Cancer (EORTC) has had a longstanding interest in sarcoma through its Soft Tissue and Bone Sarcoma Group. Other international groups that have been active in sarcoma research include the French Sarcoma Group, the Italian Sarcoma Group, the Scandinavian Sarcoma Group, the Australasian Sarcoma Group, and the Spanish Sarcoma Group (GEIS).
"More and more we are trying to coordinate these groups to perform clinical trials worldwide, which is the only way we will be able to move the field forward efficiently," Dr. Maki said. These collaborative groups are filling a gap left by bigger collaborative cancer trial groups, he added.
"Sarcoma subgroups have been cut out of the research budgets of the cooperative trials groups. The RTOG is presently the only cooperative group that still has a dedicated sarcoma committee," he said.
History of SARC The Sarcoma Alliance for Research through Collaboration was formed in 2003 by sarcoma specialists at five major medical facilities to address the lack of attention paid to the disease by NCI and other research organizations. SARC currently has a dozen clinical trials in various sarcoma subtypes. SARC's goal is to "further scientific knowledge regarding diagnosis and treatment of sarcoma, collaborate with experts to design cost-effective and efficiently executed clinical trials, and provide up-to-date information to physicians, patients, and caregivers."Visit
www.sarctrials.org/home
for more information.
An example of international collaboration is the phase II SARC study that found that R1507 monotherapy was active in patients with metastatic lesions in the Ewing's sarcoma family of tumors (American Society of Clinical Oncology [ASCO] 2010 abstract 10000).
Another pivotal player in sarcoma research is the National Cancer Institute's Cancer Therapy Evaluation Program. CTEP contracts with the pharmaceutical industry to examine specific drugs, which allows single studies involving drugs from different companies to be done.
"CTEP makes it possible to combine drugs in research studies, which is exceptionally difficult to do as an investigator-initiated study or as an industrial study because it is hard to get two industrial concerns together on one study," Dr. Maki explained. "CTEP is able to put out requests for proposals for research studies, and researchers can submit applications to test a given combination."
Dr. Maki's trial on the IGF-1R inhibitor and the mTOR inhibitor combination is a CTEP endeavor. "Kudos to CTEP for allowing us to put together this study," he said. "They have access to different companies' drugs and it does allow us to put them together for the first time. Everybody, including pharma, recognizes that this is a win-win situation."
Improving current therapy
Targeted agents are no doubt the future, but efforts are still ongoing to improve standard chemotherapy regimens for sarcoma. A study that generated buzz in the sarcoma community was the PICASSO trial, a phase II randomized, controlled trial of palifosfamide plus doxorubicin vs doxorubicin alone in patients with soft tissue sarcomas (ASCO 2010 abstract 10004).

“Sarcomas are telling us just how darn complicated all cancers are going to be. That's the message of sarcomas. They really are the canary in the coal mine.” - GEORGE DEMETRI, MD
Palifosfamide (ZIO-210) is a stabilized metabolite of ifosfamide (Ifex), but without that drug's toxicity. "Ifosfamide has been used in sarcomas since the 1980s," explained George Demetri, MD, director of the Ludwig Center and the Sarcoma Center at the Dana-Farber Cancer Institute and Harvard Medical School in Boston.
"It is a pro drug that the body has to metabolize to the active form. Different people metabolize it differently, so there is not a lot of reliability. Also, one of the metabolites of ifosfamide is toxic itself and causes bladder bleeding. Palifosfamide does not have this toxic metabolite; that is a huge advantage."
In PICASSO, combining palifosfamide with doxorubicin extended progression-free survival by a median of 3.4 months in patients with soft tissue sarcomas compared with doxorubicin alone. "In PICASSO, there was a nice signal that adding palifosfamide to regular old doxorubicin improved disease control," Dr. Demetri said. A phase III version of PICASSO has been launched with Dr. Maki and Dr. Demetri serving on the trial's board of directors.
Chemotherapy still has tremendous value in sarcoma, Dr. Demetri stressed. "I actually think that palifosfamide highlights how research is trying to use modern chemistry to make chemotherapy better," he said.
Sarcoma advocacy
BeatSarcoma
GIST Cancer Research Fund
National Leiomyosarcoma Foundation
Sarcoma Foundation of America
The Liddy Shriver Sarcoma Initiative
"I'm a huge fan of molecular targeted agents, but as cancers get more mutations and become more complicated, we are seeing a trend back to chemotherapy, because it actually works in the most mutated cancers. So maybe it's not a cure, but I would be hesitant to throw the baby away with the bathwater. If we can use the most modern chemistry to make chemotherapy safer, more tolerable, and maybe even more effective, I wouldn't be too negative," he added (see Related Reading).
An orphan no more
Sarcomas represent a true picture of the complexity of all cancers, Dr. Demetri said. "Sarcomas are telling us just how darn complicated all cancers are going to be. That's the message of sarcomas. They really are the canary in the coal mine," he said.
Of course, this newfound enthusiasm for sarcoma research has been welcomed by the oncologists who take care of these patients. "Sarcoma may have been a neglected disease 10 years ago, but there is an awful lot of research interest in sarcomas right now. The Internet and patient communities banding together have made it possible to do some of the very best, most cutting-edge research in sarcomas," he said. "The orphan status per the FDA definition is never going to go away, but the neglected orphan status is changing."
Sarcoma: A patient's perspective

BY NEIL OSTERWEIL
As a medical journalist I often use the passive voice: "The regimen was well tolerated." The reader has no way of knowing from that sentence just who is doing the tolerating, but I had the chance to find out last year when I was diagnosed with a stage III synovial sarcoma of the femur.
I live in greater Boston and have my pick of sarcoma centers, a privilege not afforded to most patients. I was also lucky to have a tumor that is sensitive to chemotherapy. After nearly a year of treatment-44 Gy of intensity-modulated radiation interleaved with three cycles of inpatient neoadjuvant chemotherapy, surgery, and three cycles of inpatient adjuvant chemotherapy, interrupted by febrile neutropenia, wound-healing problems, the red man syndrome, and infection-I am on follow-up care and hope to have put the worst behind me.
As an educated healthcare consumer, I wonder what others do when confronted with a diagnosis like mine. I attended a sarcoma oral abstract session at ASCO 2010 and heard several presenters dismiss the regimen I underwent, the MAID protocol, as too toxic. True, the anthracycline-based regimen carries risks of long-term cardiotoxicity and near-term risks such as, well, death, but isn't that the point? It's an aggressive regimen for a tumor with a high propensity for recurrence and metastasis, and despite the risks I wanted my medical team to throw everything they had at it.
I learned that some sarcoma centers use chemotherapy rarely, while others use fewer drugs for only a few cycles, because they're not convinced that any of it does any good. Hard data are hard to come by in sarcoma, but patients deserve the chance to choose the higher risk, higher reward option for themselves.
It's my tumor. Show me the data. Let me decide.
Mr. Osterweil is an award-winning freelance writer with more than 26 years of experience covering medicine and science. He can be reached at www.osterweilbaron.com.
Sarcoma Awareness Month 2023 with Brian Van Tine, MD, PhD
August 1st 2023Brian Van Tine, MD, PhD, speaks about several agents and combination regimens that are currently under investigation in the sarcoma space, and potential next steps in research including immunotherapies and vaccine-based treatments.