Intraperitoneal Chemotherapy for Advanced Epithelial Ovarian Cancer: Many Questions, Much Promise…
In 2006, after a third consecutive large-scale US phase III trial conducted by the Gynecologic Oncology Group (GOG) confirmed that use of intraperitoneal (IP) chemotherapy in optimally resected stage III epithelial ovarian cancer results in superior overall survival (OS) and/or progression-free survival (PFS),[
In 2006, after a third consecutive large-scale US phase III trial conducted by the Gynecologic Oncology Group (GOG) confirmed that use of intraperitoneal (IP) chemotherapy in optimally resected stage III epithelial ovarian cancer results in superior overall survival (OS) and/or progression-free survival (PFS),[1-3] the National Cancer Institute released a statement recommending strong consideration for using IP therapy in women with optimally cytoreduced advanced ovarian cancer.[4] Use of IP chemotherapy has not been universally accepted,[5] however, with concerns about toxicity and lack of facilities for delivery of IP infusions cited by gynecologic and medical oncologists as the primary reasons for reluctance to adopt this practice.[6] One of the major limitations of IP therapy is the increased toxicity of the regimen and increased frequency of catheter complications. Yet the most recent study, GOG172, showed impressive benefits, including an improvement in OS of 15.9 months and a 25% reduction in the risk of death,[3] along with equivalent quality of life at 1 year, compared with patients treated with intravenous (IV) therapy.[7] These are strong reasons to consider using IP therapy in a carefully selected patient population. The review in this issue of ONCOLOGY by Gonzalez et al highlights many of the critical questions, both practical and theoretical, that arise with respect to use of IP chemotherapy in the treatment of advanced epithelial ovarian cancer.[8] In this commentary we discuss the pressing questions regarding IP therapy and the current clinical trials addressing these questions.
Advantages and Current Questions
Advanced ovarian cancer spreads primarily within the peritoneal cavity and stays confined to this compartment for much of its course. Regional therapy through IP administration has several pharmacological advantages. Compared with plasma clearance, peritoneal clearance, which is dependent on molecular weight and solubility, is slower for certain drugs, and IP administration results in exposure of residual peritoneal tumor to increased concentration of drug for a prolonged period of time (higher peritoneal vs plasma AUC). Agents administered via the IP route are eventually absorbed or break down. Studies have shown similar plasma concentrations for many drugs when comparing IP versus IV therapy, while showing significantly increased peritoneal concentrations (12–1,000-fold).[9] IP delivery provides a number of pharmacologic benefits: 1) IP-administered drug will distribute directly to the predominant site of disease, the peritoneal cavity; 2) The higher concentration of drug allows for sustained exposure to a localized dose escalation beyond what can normally be achieved through the IV route; and 3) Absorption will allow for systemic distribution to extraperitoneal sites of disease.
Despite these advantages, a number of fundamental questions remain with respect to use of IP therapy in advanced epithelial ovarian cancer. For many, there is still the question of whether IP therapy is indeed advantageous compared with the current standard of care, IV therapy.[5] In an exploratory cross-trial analysis, Ozols et al reported that the difference in median OS in GOG172 (15.9 months vs 8.2 months) would have been substantially less if IV carboplatin and paclitaxel had been the control arm.[10] Ad hoc cross-trial comparisons are fraught with problems and cannot be relied upon as the basis on which to make conclusions regarding efficacy-but notably, IV carboplatin/paclitaxel was found to be noninferior, not necessarily superior, to IV cisplatin/paclitaxel (the control arm in GOG172).[11] Current studies (described in this commentary) will directly address the comparison of IV carboplatin/paclitaxel against IP chemotherapy.
Even for oncologists who have accepted IP therapy, the preferred regimen remains unclear, and more than 50% administer an adapted regimen to reduce toxicity or increase ease of administration.[6] We agree that the toxicity of IP therapy as given in GOG172 can be quite substantial. The adverse effects were generally temporary, however, improving shortly after completion of treatment, and differences in quality of life, compared with that of patients who received IV therapy, disappeared by 12 months.[7] Until the results of ongoing clinical trials are available, it is important to discuss with patients the added toxicity of the IP approach, along with the improved outcome provided IP therapy compared with by the standard treatment. What effect adaptations to the regimen-such as change in administration schedule, dose reduction, substitution of carboplatin for cisplatin, or the omission of IP paclitaxel-will have on outcomes is unclear, as they have not been tested in clinical trials. It is hoped that the current trials will provide some answers to these questions.
Another unanswered question is how much therapy needs to be delivered intraperitoneally to have an overall survival benefit. Less than 50% of patients enrolled on the IP arm of GOG172 completed the full planned schedule of 6 cycles of IP therapy.[3] Yet the intent-to-treat analysis still demonstrated a substantial survival benefit, suggesting that the greatest benefit of IP therapy may be in the first few cycles.[12] Although drug penetration is limited to superficial cell layers when delivered intraperitoneally,[9] the lower volume of disease in optimally cytoreduced disease may not require a full 6 cycles of therapy, and toxicity could potentially be diminished by a reduction in the number of cycles administered.
Other strategies for treating patients with IP therapy have been proposed. Consolidation therapy, in which IP therapy is given after successful systemic neoadjuvant therapy and interval surgical cytoreduction, has been advocated as an approach to treatment of patients with disease that is initially not surgically resectable.[13] Hyperthermic intraoperative peritoneal chemotherapy (HIPEC) and early postoperative IP chemotherapy (EPIC) along with peritonectomy have been advocated by some as an aggressive multimodality approach similar to that used in mucinous gastrointestinal cancers.[14] These approaches appear interesting but have not yet been evaluated in rigorous phase III trials. Similarly, it is unknown whether there is a role for IP therapy in patients with recurrent disease.
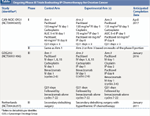
Current Phase III Trials
There are currently three phase III trials and multiple phase II trials evaluating IP therapy. Each of these trials takes a different approach to assess the role of IP chemotherapy in the treatment of ovarian cancer. The Canadian CAN-NCIC-OV21 study (NCT00993655) is a phase II/III study comparing IP plus IV chemotherapy versus IV therapy in optimally cytoreduced ovarian cancer patients following neoadjuvant IV chemotherapy. The phase II portion consists of three arms (Table 1), and one of the IP arms will be brought forward for the phase III portion of the trial. This trial will evaluate whether IP therapy results in improved PFS and OS, compared with IV therapy. It is important to note, however, that patients in this study will have been treated with neoadjuvant IV chemotherapy prior to surgical cytoreduction. The US GOG252 study (NCT00951496) is a phase III trial comparing weekly IV paclitaxel plus every-3-week IV carboplatin (Arm 1) against the same weekly IV paclitaxel plus every-3-week IP carboplatin (Arm 2) and against IV paclitaxel plus IP cisplatin and IP paclitaxel (Arm 3). It should be noted that the trial does not have a standard IV paclitaxel and carboplatin every-3-week arm. The trial is designed to address a number of issues, including the use of IP carboplatin, of a modified GOG 172 IP cisplatin regimen, and of weekly paclitaxel. The two carboplatin arms will directly compare the same dose of carboplatin given IP or IV with the same dose of weekly IV paclitaxel. Notably, bevacizumab (Avastin), given both during cytotoxic therapy and as maintenance therapy, is included in all arms of the study. It is unclear how bevacizumab, which improved the PFS benefit in GOG218,[15] will impact IP therapy.
A third phase III trial conducted through the Netherlands Cancer Institute (NCT00426257) is evaluating the role of HIPEC in secondary debulking surgeries, following either incomplete primary surgery plus adjuvant chemotherapy or primary chemotherapy administered because primary debulking surgery was not feasible.
While we look forward to the results of these studies and others with much anticipation, final analyses will not be available for several years. How should we advise our patients in the meantime? In our practice, we offer IP chemotherapy as primary adjuvant therapy to women with optimally cytoreduced ovarian cancer who have a good performance status and would be likely to tolerate this intensive therapy. Administration of IP therapy is greatly aided by the assistance of skilled and dedicated support staff. The decision to use IP chemotherapy is individualized, and the toxicities and benefits are discussed in full. While there are many questions still to be answered, we believe that IP delivery of chemotherapy is the current best approach to enhance the survival of patients with ovarian cancer.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.