Irinotecan Every 3 Weeks for Colorectal Cancer May Result in Reduced Late Diarrhea
BOSTON-Interim results from an ongoing multicenter study suggest that an irinotecan (Camptosar) dosing regimen of every 3 weeks for patients with 5-fluorouracil (5-FU)-refractory colorectal cancer may be associated with a lower incidence of grade 3-4 late diarrhea when compared to a four-times-a-week schedule. The results were presented at the 36th ASCO Annual Meeting.
BOSTONInterim results from an ongoing multicenter study suggest that an irinotecan (Camptosar) dosing regimen of every 3 weeks for patients with 5-fluorouracil (5-FU)-refractory colorectal cancer may be associated with a lower incidence of grade 3-4 late diarrhea when compared to a four-times-a-week schedule. The results were presented at the 36th ASCO Annual Meeting.
What we intend to do is compare the two most commonly used schedules for using this drug, Charles S. Fuchs, MD, study author, told ONI. The first order of business is to see whether there are any differences in efficacy between the two schedules. We dont have any data on that yet.
In addition, we want to see what differences there are in toxicity and quality of life, said Dr. Fuchs, of the Dana-Farber Cancer Institute and Harvard Medical School.
This study, which is still accruing patients, will directly compare two regimens of irinotecan in a total of 300 patients with 5-FU-refractory colorectal cancer. These regimens are commonly used in the United States for second-line therapy of colorectal cancer after first-line 5-FU.
Regimen A delivers irinotecan at 125 mg/m² four times a week for 6 weeks. In regimen B, patients receive 350 mg/m² every 3 weeks. Patients 70 years of age or older, with a performance status of 2, or with prior pelvic irradiation, receive 300 mg/m².
Patients were given supportive care, which included standard antiemetics, atropine for cholinergic symptoms, and loperamide for late diarrhea.
Preliminary Results
Results of the study to date include data on 40 patients in arm A and 82 patients in arm B.
Patient characteristics in both arms are comparable and include a median age of 65 in arm A and 63 years in arm B, with 33% in arm A and 26% in arm B being 70 or older. Sixty percent of patients in arm A are male, compared with 56% in arm B. For arm B, starting doses of 350 mg/m² were given in 56% of this group, and 46% received 300 mg/m².
Clinically relevant grade 3-4 adverse events in the first 6 weeks of therapy for patients in cycle 1 of arm A vs cycles 1 and 2 of arm B are shown in the Table.
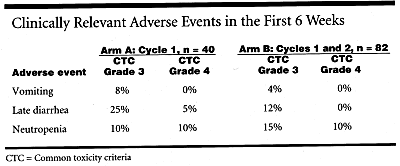
Additionally, moderate-to-severe cholinergic symptoms occurred in 2.5% of arm A patients vs 28% of arm B patients.
Final analyses, which may occur as early as summer 2000, will directly compare 1-year survival, time to tumor progression, response rate, safety, and quality of life among all 300 patients.