Irinotecan Proves Beneficial in Heavily Pretreated Advanced Breast Cancer Patients
JACKSONVILLE, Florida-An encouraging study reported at the 38th Annual Meeting of the American Society of Clinical Oncology (abstract 206) suggests that irinotecan (CPT-11, Camptosar) may prove valuable in advanced breast cancer.
JACKSONVILLE, FloridaAn encouraging study reported at the 38th Annual Meeting of the American Society of Clinical Oncology (abstract 206) suggests that irinotecan (CPT-11, Camptosar) may prove valuable in advanced breast cancer. The randomized multicenter phase II study by the North Central Cancer Treatment Group was spearheaded by Edith Perez, MD, professor of medicine, Mayo Clinic, Jacksonville, Florida.
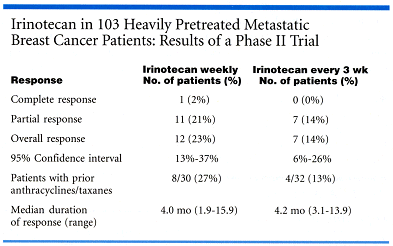
The study included 103 heavily pretreated metastatic breast cancer patients with visceral and soft tissue metastases. Over half of the patients had been pretreated with both anthracyclines and taxanes, and about one third had received two treatment regimens for metastatic disease.
The patients received irinotecan as a single agent either on a weekly basis (100 mg/m² for 4 weeks with 2 weeks off) or 240 mg/m² every 3 weeks. Treatment was continued until disease progression.
Irinotecan has not been properly evaluated for its activity in breast cancer. Dr. Perez said that, to her knowledge, this study provides the first data by a North American cooperative group on a topoisomerase inhibitor in breast cancer.
"The response rates were very interesting, especially in patients who had received both anthracyclines and taxanes. In the every-3-week arm, we achieved a respectable 13% response rate, and in the other arm, 27%. So we could speculate that if we have this high of a response rate after anthracyclines and taxanes, it could be a lot higher in earlier stage breast cancer," she said.
Median time to progression was comparable to that seen with other single agents, which is about 3 to 4 months (see table below), Dr. Perez said.
Treatment produced an acceptable level of toxicity. Grade 3-4 neutropenia occurred in 32% of patients treated weekly and in 36% of those treated every 3 weeks; diarrhea occurred in 17% and 12%; nausea in 6% and 16%; vomiting in 4% and 20%; and dyspnea in 14% and 18%, respectively.
A randomized trial is being designed to determine whether irinotecan should be positioned before or after capecitabine (Xeloda), following therapy with anthracyclines and taxanes. The third arm will be the combination of irinotecan/capecitabine.