Irradiation in Early-Stage Breast Cancer: Conventional Whole-Breast, Accelerated Partial-Breast, and Accelerated Whole-Breast Strategies Compared
Given their greater convenience and, in most cases, decreased costs, APBI and AWBI are becoming increasingly popular alternatives to conventional WBI for early-stage breast cancer patients who desire BCT. However, given the protracted time to local recurrence and complications following BCT, definitive results from randomized clinical trials comparing conventional WBI vs AWBI or APBI are limited.
Whole-breast irradiation (WBI) following breast-conserving surgery (BCS) has been used for several decades as an alternative to mastectomy in the treatment of localized breast cancer, and it has been shown to decrease rates of local-regional recurrence and improve survival rates compared with BCS alone. WBI is delivered using high-energy external beam radiation and typically consists of approximately 5 weeks of daily treatments to the entire breast, with or without inclusion of a “boost” to the primary site. Accelerated partial-breast irradiation (APBI) and accelerated whole-breast irradiation (AWBI) have been developed as alternatives to conventional WBI for selected patients with early-stage breast cancer. Given its large size and long follow-up, the Canadian trial of AWBI has been widely considered as practice-changing, and additional studies of AWBI are maturing or newly-launched. Use of APBI is based on the observation that the majority of local recurrences occur near the primary tumor site. Because a smaller portion of the breast is irradiated, treatment courses can often be abbreviated, and techniques such as conformal external beam, catheter- or balloon-based brachytherapy, and intra-operative radiation have been developed. Data from early APBI randomized trials and retrospective studies report mixed results. However, given the protracted time to local recurrence and complications following breast-conserving therapy, definitive results from contemporary randomized clinical trials comparing conventional WBI against AWBI or APBI are still limited.
Whole-Breast Irradiation (WBI)
Today, many women with early-stage invasive breast cancer are candidates for breast-conserving therapy (BCT), which consists of breast-conserving surgery (BCS) followed by radiation to all or a portion of the treated breast. The goal of BCT is to provide oncologic outcomes equivalent to those of mastectomy while preserving a cosmetically acceptable breast. Mature data from several large prospective randomized trials have shown that overall survival is equivalent with BCT or mastectomy.[1-3]
Results from randomized trials comparing BCS with or without WBI have been analyzed by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Whereas the individual trials did not have the statistical power to detect improved survival with the addition of radiation, the EBCTCG meta-analysis demonstrated that the improvement in local control provided by radiation translated into an overall survival benefit.[4,5] The original analysis showed that radiation provided similar proportional reductions in local recurrence in all subgroups of patients, and that prevention of local recurrence at 5 years translated into improved survival at 15 years, at an approximate 4:1 ratio.[4]
In the most recent EBCTCG update, 7 additional trials in low-risk patients were added, follow-up was obtained in the initial 10 trials and, importantly, local and distant recurrences were analyzed together to determine the effect of radiation on 10-year “first recurrence” and 15-year breast cancer death rates.[5] (Technically, the probabilities of local and distant recurrence are not statistically independent, and therefore valid estimates of the separate effects of radiation on local and distant recurrence cannot be obtained.[6]) Overall, WBI reduced the 10-year risk of any first recurrence with a rate ratio (RR) of about half (RR = 0.52), from 35.0% to 19.3% (2P < .00001), and it reduced the 15-year risk of breast cancer death by about one-sixth (RR = 0.82), from 25.2% to 21.4% (2P = .00005). The risk of death from any cause was similarly lower in the radiation arm (34.6% vs 37.6%; 2P = .03), indicating that WBI does not significantly increase non–breast cancer deaths.
The EBCTCG analysis shows the benefit of radiation in preventing recurrence is largest in the first year, but remains substantial throughout the first decade, whereas the benefit in breast cancer deaths becomes apparent only after several years and continues well into the second decade. (Interestingly, 5 years of tamoxifen therapy also reduced the annual rate of any recurrence over the first 10 years by about one-half [RR = 0.53], but reduced the annual rate of breast cancer death by about one-third [RR = 0.68].[7]) The risk reduction in recurrence and death with radiation was present for women with both negative and positive lymph nodes. Benefit was seen in patients of all ages, and with various tumor grades and sizes, although the absolute magnitude of the benefit varied. Based on the data from this update, the new “4:1 ratio” is between the reduction in first recurrence at 10 years (as opposed to the reduction in local recurrence at 5 years) and the reduction in mortality at 15 years.
The rates of ipsilateral breast tumor recurrence (IBTR) with BCS and WBI have been reduced considerably over time, and the 5-year rate is now about 2%.[8] The reasons for this improvement are several-fold and include better imaging with mammography, more thorough pathologic evaluation of the resected specimens (especially margin status), and improvements in systemic therapy that, when combined with radiation, substantially reduce IBTR.[9]
WBI is well tolerated. Long-term cosmetic outcomes following WBI are quite good, and significant treatment-associated morbidity is rare. In a series of more than 400 patients with stage I/II breast cancer treated with WBI, the rate of unacceptable cosmetic results was 6.7% at 11 years, and was primarily limited to patients who received doses higher than 60 Gy.[10] Rates of other complications-including rib fracture, pericarditis, and tissue necrosis-have also been shown to be quite low, and these risks are likely further decreased with the use of current techniques.[11]The time courses for both local recurrence and treatment complications are long, so long-term follow-up is needed to assess efficacy and safety. There is now extensive long-term experience worldwide using WBI.
WBI Techniques
Contemporary WBI begins with CT-based planning. Patients are typically treated supine with arms above the head. Other positions, such as prone or lateral decubitus, can be useful for patients with large or pendulous breasts. Left-sided tumors can be treated using a heart block or breath-holding techniques to avoid direct heart irradiation, but significant patient cooperation and special in-room patient-position monitoring are required for the breath-hold technique.
WBI is usually delivered via tangent fields using high-energy x-rays. Forward planning techniques allow addition of sub-fields to optimize dose homogeneity; intensity-modulated radiotherapy (IMRT) is typically not required. Standard tangent fields cover a substantial percentage of level I and II axillary nodes. “High tangent” techniques can be used to treat a greater percentage of the axilla. Treatments are delivered daily to the entire breast in 1.8- to 2-Gy fractions, with total whole-breast doses of 45 to 50 Gy. Two large randomized trials have investigated the impact of a 10 or 16 Gy boost dose to the primary site following BCS and WBI, and both showed that addition of a boost significantly decreased local recurrence rates (from 10.2% to 6.2% at 10 years in the EORTC [European Organisation for Research and Treatment of Cancer] trial and from 4.5% to 3.6% at 5 years in the Lyon trial).[12,13] Provider-assessed rates of fibrosis and telangiectasias were increased with addition of a boost, but patient-reported cosmetic outcomes did not differ.
Accelerated Whole-Breast Irradiation (AWBI)
Conventional WBI is typically delivered over a period of about 5 weeks. Recently, there has been increased interest in accelerated courses of WBI.[14] Accelerated whole breast irradiation (AWBI) shortens the length of the treatment course and has become more feasible due to two major developments: technical improvements in the delivery of breast irradiation that result in a much higher level of dose homogeneity, and a better understanding of the biologic equivalence of accelerated and conventional dose schedules.
TABLE 1
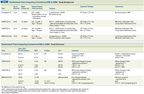
Randomized Trials Comparing Conventional WBI to AWBI: Study Background
Several large randomized trials have investigated AWBI in the treatment of early breast cancer (Table 1).[15-18] In each of these trials, hypofractionated schedules were compared against conventional WBI of 50 Gy in 2-Gy daily fractions. Doses in the experimental arms varied from 39 Gy in 13 fractions in the UK Royal Marsden Hospital/Gloucestershire Oncology Centre (RMH/GOC) and UK Standardisation of Breast Radiotherapy (START) Trial A trials, to 42.5 Gy in 16 fractions in the Canadian trial. No significant differences in overall local control rates between conventional and hypofractionated arms were noted in any trial. Given its large size and long follow-up, the Canadian trial in particular has been widely considered to be practice-changing. In an exploratory subset analysis, women with grade 3 tumors had a 15.6% rate of IBTR in the hypofractionated arm of the Canadian trial vs 4.7% in the conventional fractionation arm (P = .01). This difference was not seen in a large retrospective patient population.[19] Additional data and longer follow-up will be needed to determine whether any patient or tumor factors are associated with increased risk of IBTR following AWBI compared with conventional WBI.
The cosmetic implications of AWBI were also assessed in these trials and were quite favorable. Late breast changes were not significantly different between conventional and hypofractionated arms.[20] Provider- and patient-assessed adverse cosmetic outcomes were significantly higher with 50 Gy than 39 Gy (P = .01) in START A, and significantly higher with 50 Gy than 40 Gy (P = .02) in START B. In a separate analysis, rates of other radiation-related adverse effects were lower for the 39-Gy regimen in START A and 40-Gy regimen in START B compared with the 50-Gy control arms.[21] Continued follow-up will be needed to determine how long-term cosmetic effects and toxicity of AWBI compare with those of conventional WBI.
Based on the available data, a task force of the American Society for Radiation Oncology (ASTRO) has developed guidelines for the use of AWBI.[22] The task force favored a dose schedule of 42.5 Gy in 16 fractions (“Canadian”) and its use in patients aged 50 years or older with pT1–2N0 cancer treated with BCS and not treated with adjuvant chemotherapy, where the dose homogeneity is within +/− 7% and the heart can be excluded from direct irradiation. There was no agreement on the use of a boost.
Additional follow-up data are being obtained on the first-generation AWBI trials, and multiple second-generation AWBI trials have already been launched. Trial designs vary, and various schedules of AWBI (with or without a sequential or concurrent boost) are being compared against conventional WBI or accelerated partial-breast irradiation (APBI). For example, the Radiation Therapy Oncology Group (RTOG) 10-05 trial is currently enrolling early-stage patients and randomizing them to conventional (or “Canadian” hypofractionation) WBI with a sequential boost or to AWBI with a concurrent boost. It is likely that the results of these trials will expand the accepted indications and dose schedules for AWBI.
Rationale for APBI
Although WBI remains the most common technique for delivery of radiation following BCS, APBI has been employed for many years, and interest surrounding its use continues to grow. The rationale for APBI stems from the observation that the majority of breast cancer recurrences occur near the primary tumor site.[23-26]Although multicentric disease can be found in a proportion of mastectomy specimens, the rate of breast cancer diagnosed outside the vicinity of the surgical cavity following WBI may not be significantly different than that for an unirradiated or contralateral breast.[27,28] Therefore, confining radiation to the area immediately surrounding the tumor may provide equivalent rates of primary tumor control, while sparing radiation to regions that are at low risk of harboring clinically relevant microscopic disease. APBI can also potentially minimize the dose to adjacent normal structures, including the heart, lungs, ribs, and soft tissues, which could reduce the risk of radiation-induced late complications.
Because less total tissue is irradiated, higher daily doses can be delivered over fewer fractions. Fewer treatment visits may improve patient satisfaction and quality of life by minimizing the psychological and physical strain associated with treatment.[29,30] Shorter courses of therapy could also improve compliance with radiation in elderly and geographically isolated patients, populations shown to have lower compliance with radiation following BCS.[31-33] Finally, some forms of APBI could improve efficiency and decrease the cost of treatment.[34,35]
APBI Techniques and Non-randomized Experiences
Several techniques have been developed to deliver APBI. Although the modalities vary significantly, all are designed to deliver therapeutic doses to the tissue near the surgical cavity that is believed to be at highest risk of recurrence.
External beam radiation techniques similar to those used for WBI have been adapted to deliver APBI. These techniques have the advantage of being noninvasive and can utilize many of the same treatment planning and delivery tools as WBI. Typical doses are 36 to 38.5 Gy in 10 fractions delivered twice daily over a period of 5 days. Conformal 3D-RT or IMRT planning can be used, and a variety of beam arrangements have been described. Early results from a number of institutional reports appear to be favorable.[36,37]RTOG 0319, a phase I/II trial with 58 patients, showed an IBTR rate of 6% (4% within the treatment field), and 2 patients with grade III skin toxicity at 4.5 years.[38] Toxicity analysis of a randomized trial comparing conventional WBI vs IMRT-based APBI showed lower rates of acute skin toxicity in the APBI arm.[39] No clear dose–toxicity relationship has been identified, and although initial results are promising, long-term follow-up is lacking.
Interstitial brachytherapy using multiple catheters and high-dose rate (HDR) or low-dose rate (LDR) sources was originally developed to deliver a boost dose to the surgical cavity following WBI, but has also been adapted to deliver APBI. The number and position of catheters are determined by the size and shape of the surgical cavity. Once inserted, the catheters are loaded at predetermined locations, to deliver the target dose to the breast tissue immediately surrounding the surgical cavity. Iodine-125 sources are typically used for LDR delivery and are prescribed to be delivered to a total dose of 45 to 50 Gy. Iridium-192 is the most common HDR source and is prescribed to 34 Gy, typically given over 10 fractions (twice daily for 5 days). Because of the steep dose falloff, interstitial brachytherapy allows for rapid delivery of high radiation doses to target tissues with nearly complete sparing of surrounding normal structures. However, due to the invasive nature of the procedure, infection, fat necrosis, or scarring can occur.
Several interstitial brachytherapy experiences in early-stage breast cancer have been published. RTOG 95-17 enrolled 100 stage I/II breast cancer patients who were treated with catheter-based HDR or LDR brachytherapy. IBTR rates for HDR and LDR techniques were 3% and 6%, respectively.[40] A separate toxicity analysis revealed two grade 3-4 toxicities with HDR and three grade 3-4 toxicities with LDR.[41] The 10-year cumulative incidence of IBTR in a series of patients treated with interstitial brachytherapy at William Beaumont Hospital was 5%, with a matched-pair analysis showing outcomes similar to those of patients treated with WBI.[42] The 5-year rate of fat necrosis in these patients was 11%, but 95% to 99% of cosmetic outcomes were reported as good to excellent.[43] However, 12-year results from a series of 50 patients treated with LDR interstitial brachytherapy showed six cases of IBTR (12%), somewhat lower rates of acceptable cosmesis (67% good to excellent results, 54% moderate to severe fibrosis), and more treatment-related toxicity with longer follow-up.[44]
Intracavitary brachytherapy is an alternative brachytherapy technique that can be used to deliver APBI. The most commonly used intracavitary device is the MammoSite applicator, which was approved by the US Food and Drug Administraion (FDA) in 2002. The device is inserted into the lumpectomy cavity several days following surgery (after pathologic confirmation of margin status) and inflated. A CT scan is obtained for treatment planning, and iridium-192 is afterloaded into a single lumen in the center of the balloon to deliver the prescribed dose at the surface of the lumpectomy cavity surrounding the balloon. Alternate devices with multiple lumens are also available and allow for greater flexibility in treatment planning. A dose of 34 Gy is delivered in 3.4 Gy fractions given twice daily over a period of 5 days. Following treatment, the balloon is deflated and removed. Advantages of intracavitary brachytherapy include its ease of use compared with interstitial techniques and its reproducibility in delivery of radiation dose to the balloon surface. However, problems with dose homogeneity can occur when the surgical cavity is irregularly shaped, and treatment of superficial cavities can lead to higher skin dose and increased toxicity. The 5-year rate of IBTR in more than 1400 patients enrolled on the MammoSite registry is 3.8%, with good-to-excellent cosmetic results reported in 90.4%.[45] Two-year data from a multi-institutional series of 483 patients treated using the MammoSite applicator show a 1.6% IBTR rate and 90% good-to-excellent cosmetic outcomes.[46] A recent population-based retrospective analysis of 92,735 older women treated with WBI or brachytherapy-based APBI showed a significantly increased incidence of subsequent mastectomy as well as higher rates of post-operative complications, breast pain, fat necrosis, and rib fracture in patients treated with brachytherapy.[47]
Intraoperative radiation is another technique for delivery of APBI, and is administered in a single fraction to the lumpectomy cavity immediately following tumor removal. One technique, TARGIT (TARGeted Intraoperative radioTherapy), employs low-energy x-rays emitted from a source located at the center of a spherical applicator placed within the surgical cavity. The prescription dose of 20 Gy at 0.2 cm depth and 5 Gy at 1 cm depth is delivered over a period of several minutes, after which time the applicator is removed and the surgical incision is closed. The technique has been criticized for not delivering adequate dose to a sufficient margin around the cavity. Another technique, ELIOT (ELectron beam Intraoperative radioTherapy), employs a dedicated linear accelerator in the operating room to deliver electron beam radiation. Although not widely practiced in the US, intraoperative radiation has the advantage of being completed in a single day and treats the operative bed in its native state prior to surgical closure. In a series of more than 1800 women treated with quadrantectomy followed by intraoperative radiation with electrons, the rates of local recurrence and new primary ipsilateral cancers at 36 months were 2.3% and 1.3%, respectively, while rates of fat necrosis and fibrosis were 4.2% and 1.8%, respectively.[48] A disadvantage of intraoperative radiation is that pathologic information regarding margin status and lymph node involvement are not available at the time of treatment. If unfavorable pathologic features are found, subsequent WBI can be administered. When used as a boost prior to planned post-operative WBI, intraoperative delivery of 20 Gy to the surgical cavity was associated with a 5-year IBTR rate of 1.7%.[49]
Randomized Trials Comparing APBI vs WBI
TABLE 2

Randomized Trials Comparing APBI to WBI: Study Background
Four randomized trials have been published that compared conventional WBI vs APBI (Table 2). The first published trial was conducted at Christie Hospital in Manchester, UK and randomized more than 700 women to receive external beam APBI to the surgical cavity or an accelerated course of WBI.[50,51] With follow-up of 8 years, there was no difference in overall or disease-specific survival, but IBTR was significantly higher in the APBI arm compared with the WBI arm (25% vs 13%; P = .00008). The IBTR rate was 22% in the APBI arm vs 12% in the WBI arm for women with invasive ductal carcinoma, and 43% in the APBI arm vs 17% in the WBI arm for patients with invasive lobular carcinoma. The results of the trial suggest superior local control with WBI, but several factors limit its applicability. Microscopic margin status was not evaluated in these patients and axillary lymph node staging was not performed. The doses used for WBI are significantly lower than those typically used today, and no boost was given. Many patients had poor prognostic factors, including large tumor size, nonductal histology, high grade, and the presence of lymphovascular invasion that today would prompt more aggressive therapy.
The second published randomized APBI experience is from Leeds Hospital in the UK.[52] As in the Christie Hospital trial, women were randomized to external beam APBI or an accelerated course of WBI. At 8 years median follow-up, there were 4 IBTRs in the WBI arm compared with 10 in the APBI arm (P = .07), and 4 isolated axillary recurrences in the WBI arm compared with 10 in the APBI arm (P = .05). Once again, the lack of microscopic margin status severely limits the findings of this trial. APBI patients were treated with a variety of techniques, and no clear definition for field size was used.
A randomized trial comparing conventional WBI vs APBI was also conducted in Hungary.[53] The majority of APBI patients underwent multi-catheter Ir-192 brachytherapy, while a smaller percentage who were technically unsuited for brachytherapy were treated using external beam APBI. At a median follow-up of 66 months, IBTR as a first event occurred in six patients (4.7%) in the APBI arm and four patients (3.1%) in the WBI arm. In the APBI arm, two of the six IBTRs were in the treated volume or its margin. The rate of good-to-excellent cosmetic results was 77.6% in the APBI arm and 62.9% in the WBI arm (P = .009). In a separate publication, the reported rates of asymptomatic or symptomatic fat necrosis at 4 years did not differ between WBI and APBI patients.[54] The trial is limited by the relatively small number of patients, short follow-up, and variability in treatment within each arm across institutions.
The most recent published trial comparing conventional WBI vs APBI is the TARGIT-A trial.[55] APBI patients underwent wide local excision plus sentinel lymph node biopsy or axillary dissection followed by targeted intraoperative radiotherapy (TARGIT) to a prescribed surface dose of 20 Gy. Fourteen percent of patients treated with TARGIT had adverse pathologic features on final pathology and subsequently underwent WBI. At 4 years, there were six IBTRs in the TARGIT arm vs five in the WBI arm, and four axillary recurrences in the TARGIT arm vs three in the WBI arm. The number of patients with major toxicity was similar between arms; however, the types of complications varied. Seroma requiring three or more aspirations occurred more frequently in the TARGIT arm (2.1% vs 0.8%; P = .012), while RTOG grade 3 toxicity was more common in the WBI arm (2.1% vs 0.5%; P = .002).
The results of three of these APBI trials have been analyzed in a recent meta-analysis.[56] Although enrollment criteria and APBI techniques vary widely across studies, no difference in survival was noted in patients treated with APBI vs WBI (P = .55). APBI was associated with a higher risk of IBTR (odds ratio [OR] 2.15; 95% confidence interval [CI], 1.40-3.31) and axillary recurrence (OR, 3.43; 95% CI, 2.06-5.72).
Consensus Guidelines and Use of APBI
Given the expanding use of APBI in the treatment of early-stage breast cancer, task forces representing several professional societies have published consensus statements regarding its usage. ASTRO reviewed the available literature and reached a consensus regarding categories of patients for whom APBI is deemed “suitable,” “cautionary,” or “unsuitable.”[57] Suitable patients are those 60 years of age and older with small, unifocal tumors of ductal or other favorable histologic subtype without nodal involvement who have undergone complete surgical excision of their tumors, to negative margins, and have not received neoadjuvant chemotherapy. Only patients in the suitable category are recommended to undergo APBI outside of a clinical trial. Cautionary patients are those with larger tumors, less favorable histology, an extensive intraductal component (EIC), pure ductal carcinoma in situ (DCIS), or close surgical margins. Young patients or those with large tumors, positive margins, unfavorable pathologic features, or involved lymph nodes are considered unsuitable candidates for APBI. There is ongoing debate about whether more groups of patients should be considered suitable. Because of the variability of technical factors and the short available follow-up, recommendations regarding APBI techniques and treatment planning were not addressed by the ASTRO committee.
The American Brachytherapy Society (ABS) Breast Brachytherapy Task Group recommends limiting APBI with interstitial or intracavitary brachytherapy to patients 50 years of age and older with invasive ductal tumors measuring 3 cm or less and no nodal involvement.[58] Multifocal disease and an EIC are considered relative contraindications.
Despite the recommendations of such task forces, APBI usage and techniques vary widely. A 2011 analysis of Medicare data suggests that use of brachytherapy following BCS has increased from less than 1% of new breast cancer cases in 2001 to 10% of cases in 2006. This increase has correlated with FDA approval of MammoSite and its reimbursement by Medicare.[59] A similar analysis of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database shows that the percentage of women receiving brachytherapy-based APBI increased from 0.4% in 2000 to 6.6% in 2007.[60] In the study, 65.8% of treated patients were classified as cautionary or unsuitable based on ASTRO criteria.
Although the use of APBI is increasing, the growth in APBI is variable across patient demographics and regions. Brachytherapy-based APBI is more common among Caucasian patients and in those with non-HMO (health maintenance organization) insurance. Metropolitan regions, regions with higher median income, and regions with a lower density of radiation oncologists are also more likely to have higher rates of APBI.[59,60]
TABLE 3

Ongoing APBI Trials
Ongoing Studies
Several randomized trials comparing APBI vs WBI are ongoing (Table 3). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/RTOG 0413 trial is the largest of these investigations and has nearly completed randomization of 4300 post-lumpectomy patients to conventional WBI or APBI using multi-catheter brachytherapy, balloon catheter brachytherapy, or external beam radiation. Randomized trials are also underway in other countries and compare APBI against conventional WBI or AWBI. Although many of these trials are accruing briskly, and data on early toxicity may be available in the next several years, many more years will be required before data regarding long-term efficacy and safety are available. In total, more than 14,000 patients have been accrued to randomized phase III trials of APBI (compared with fewer than 4100 patients in the trials that established the equivalence of BCT to mastectomy), so definitive results should become available with time.
In the personal perspective of the senior author (JH) who was involved in the early use, and controversies regarding use, of BCS and WBI as an alternative to mastectomy:
• Those controversies, often intense, dissipated only when definitive results of randomized clinical trials became available. In the current situation, in which definitive results from trials comparing conventional WBI, accelerated WBI, and APBI are not available, there will inevitably be controversy where experts will differ in their opinions.
• The time course to assess efficacy and safety in local treatment of the breast using radiation is protracted, and many early assessments of BCS and WBI underestimated late IBTRs and side effects. This long-term follow-up, sometimes painful to see, provided information that helped to insure that future implementation of BCS and WBI would be safer and more effective.
Conclusions
Given their greater convenience and, in most cases, decreased costs, APBI and AWBI are becoming increasingly popular alternatives to conventional WBI for early-stage breast cancer patients who desire BCT. However, given the protracted time to local recurrence and complications following BCT, definitive results from randomized clinical trials comparing conventional WBI vs AWBI or APBI are limited. The results of the Canadian hypofractionation trial have been widely considered to be practice-changing, and forthcoming results of other first- and second-generation AWBI trials will continue to guide the expanded used of AWBI. Mature data for APBI is less robust, but results from several non-randomized studies suggest that, in carefully selected patients, APBI can provide rates of local control similar to those achieved with conventional WBI. However, a recent large retrospective analysis suggests that brachytherapy-based APBI may be inferior to WBI in certain populations. Results from published randomized trials comparing APBI vs WBI are also mixed, and data from the large ongoing randomized trials using contemporary techniques are needed to further characterize the safety and efficacy of APBI. Until mature randomized data are available, patients should be counseled that long-term results of APBI are limited, and providers should be judicious in their use of APBI outside of consensus guidelines or a clinical trial setting.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-41.
2. van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143-50.
3. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:
1227-32.
4. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-106.
5. Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707-16.
6. Gelman R, Gelber R, Henderson IC, et al. Improved methodology for analyzing local and distant recurrence. J Clin Oncol. 1990;8:548-55.
7. Early Breast Cancer Trialists’ Collaborative Group, Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771-84.
8. Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885-91.
9. Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer--bigger is not better. N Engl J Med. 2012;367:79-82.
10. Delouche G, Bachelot F, Premont M, et al. Conservation treatment of early breast cancer: long term results and complications. Int J Radiat Oncol Biol Phys. 1987;13:29-34.
11. Pierce SM, Recht A, Lingos TI, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:915-23.
12. Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259-65.
13. Romestaing P, Lehingue Y, Carrie C, et al. Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997;15:963-8.
14. Schoenfeld JD, Harris JR. Abbreviated course of radiotherapy (RT) for breast cancer. Breast. 2011;20(Suppl 3):S116-27.
15. Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331-41.
16. Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098-107.
17. Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467-71.
18. Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513-20.
19. Herbert C, Nichol A, Olivotto I, et al. The impact of hypofractionated whole breast radiotherapy on local relapse in patients with grade 3 early breast cancer: a population-based cohort study. Int J Radiat Oncol Biol Phys. 2012;82:2086-92.
20. Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75:9-17.
21. Hopwood P, Haviland JS, Sumo G, et al. Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncol. 2010;11:231-40.
22. Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81:59-68.
23. Fowble B, Solin LJ, Schultz DJ, et al. Breast recurrence following conservative surgery and radiation: patterns of failure, prognosis, and pathologic findings from mastectomy specimens with implications for treatment. Int J Radiat Oncol Biol Phys. 1990;19:833-42.
24. Kurtz JM, Amalric R, Brandone H, et al. Local recurrence after breast-conserving surgery and radiotherapy. Frequency, time course, and prognosis. Cancer. 1989;63:1912-7.
25. Smith TE, Lee D, Turner BC, et al. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281-9.
26. Fisher ER, Anderson S, Redmond C, et al. Ipsilateral breast tumor recurrence and survival following lumpectomy and irradiation: pathological findings from NSABP protocol B-06. Semin Surg Oncol. 1992;8:161-6.
27. Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985;56:979-90.
28. Vaidya JS, Vyas JJ, Chinoy RF, et al. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer. 1996;74:820-4.
29. Whelan TJ, Levine M, Julian J, et al. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;
88:2260-6.
30. Kawase E, Karasawa K, Shimotsu S, et al. Estimation of anxiety and depression in patients with early stage breast cancer before and after radiation therapy. Breast Cancer. 2012;19:147-52.
31. Athas WF, Adams-Cameron M, Hunt WC, et al. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. 2000;92:269-71.
32. Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326:1097-101.
33. Lazovich DA, White E, Thomas DB, et al. Underutilization of breast-conserving surgery and radiation therapy among women with stage I or II breast cancer. JAMA. 1991;266:3433-8.
34. Lievens Y. Hypofractionated breast radiotherapy: financial and economic consequences. Breast. 2010;19:192-7.
35. Suh WW, Pierce LJ, Vicini FA, et al. A cost comparison analysis of partial versus whole-breast irradiation after breast-conserving surgery for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2005;62:790-6.
36. Chen PY, Wallace M, Mitchell C, et al. Four-year efficacy, cosmesis, and toxicity using three-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2010;76:991-7.
37. Berrang TS, Olivotto I, Kim DH, et al. Three-year outcomes of a canadian multicenter study of accelerated partial breast irradiation using conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2011;
81:1220-7.
38. Vicini F, Winter K, Wong J, et al. Initial efficacy results of RTOG 0319: three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/ II breast carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1120-7.
39. Livi L, Buonamici FB, Simontacchi G, et al. Accelerated partial breast irradiation with IMRT: new technical approach and interim analysis of acute toxicity in a phase III randomized clinical trial. Int J Radiat Oncol Biol Phys. 2010;77:509-15.
40. Arthur DW, Winter K, Kuske RR, et al. A phase II trial of brachytherapy alone after lumpectomy for select breast cancer: tumor control and survival outcomes of RTOG 95-17. Int J Radiat Oncol Biol Phys. 2008;72:467-73.
41. Kuske RR, Winter K, Arthur DW, et al. Phase II trial of brachytherapy alone after lumpectomy for select breast cancer: toxicity analysis of RTOG 95-17. Int J Radiat Oncol Biol Phys. 2006;65:45-51.
42. Antonucci JV, Wallace M, Goldstein NS, et al. Differences in patterns of failure in patients treated with accelerated partial breast irradiation versus whole-breast irradiation: a matched-pair analysis with 10-year follow-up. Int J Radiat Oncol Biol Phys. 2009;74:447-52.
43. Chen PY, Vicini FA, Benitez P, et al. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation: a method of radiation delivery by interstitial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;
106:991-9.
44. Hattangadi JA, Powell SN, Macdonald SM, et al. Accelerated partial breast irradiation with low-dose-rate interstitial implant brachytherapy after wide local excision: 12-year outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83:791-800.
45. Vicini F, Beitsch P, Quiet C, et al. Five-year analysis of treatment efficacy and cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:808-17.
46. Cuttino LW, Keisch M, Jenrette JM, et al. Multi-institutional experience using the MammoSite radiation therapy system in the treatment of early-stage breast cancer: 2-year results. Int J Radiat Oncol Biol Phys. 2008;71:107-14.
47. Smith GL, Xu Y, Buchholz TA, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307:1827-37.
48. Veronesi U, Orecchia R, Luini A, et al. Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons. Breast Cancer Res Treat. 2010;124:141-51.
49. Vaidya JS, Baum M, Tobias JS, et al. Long-term results of targeted intraoperative radiotherapy (Targit) boost during breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2011;81:1091-7.
50. Magee B, Swindell R, Harris M, et al. Prognostic factors for breast recurrence after conservative breast surgery and radiotherapy: results from a randomised trial. Radiother Oncol. 1996;39:223-7.
51. Ribeiro GG, Magee B, Swindell R, et al. The Christie Hospital breast conservation trial: an update at 8 years from inception. Clin Oncol (R Coll Radiol). 1993;5:278-83.
52. Dodwell DJ, Dyker K, Brown J, et al. A randomised study of whole-breast vs tumour-bed irradiation after local excision and axillary dissection for early breast cancer. Clin Oncol (R Coll Radiol). 2005;17: 618-22.
53. Polgar C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma--5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694-702.
54. Lovey K, Fodor J, Major T, et al. Fat necrosis after partial-breast irradiation with brachytherapy or electron irradiation versus standard whole-breast radiotherapy--4-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:724-31.
55. Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91-102.
56. Valachis A, Mauri D, Polyzos NP, et al. Partial breast irradiation or whole breast radiotherapy for early breast cancer: a meta-analysis of randomized controlled trials. Breast J. 2010;16:245-51.
57. Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys. 2009;74:987-1001.
58. Arthur DW, Vicini FA, Kuske RR, et al. Accelerated partial breast irradiation: an updated report from the American Brachytherapy Society. Brachytherapy. 2003;2:124-30.
59. Smith GL, Xu Y, Buchholz TA, et al. Brachytherapy for accelerated partial-breast irradiation: a rapidly emerging technology in breast cancer care. J Clin Oncol. 2011;29:157-65.
60. Hattangadi JA, Taback N, Neville BA, et al. Accelerated partial breast irradiation using brachytherapy for breast cancer: patterns in utilization and guideline concordance. J Natl Cancer Inst. 2012; 104:29-41.