It’s Time for Clinicians to Reconsider Their Proscription Against the Use of Soyfoods by Breast Cancer Patients
Recently published research questions the need for the advised restriction against the use of soyfoods by women with a history of breast cancer.
The impact of soyfood intake on breast cancer risk has been intensely investigated. This focus can be attributed to soyfoods being uniquely rich dietary sources of isoflavones. Isoflavones are classified as both phytoestrogens and selective estrogen receptor (ER) modulators. The finding that dietary genistein, the primary soybean isoflavone, stimulates the growth of existing mammary tumors in ovariectomized athymic mice implanted with ER-positive breast cancer cells has led many oncologists to advise their patients against the use of soyfoods. However, the clinical evidence indicates that isoflavone exposure has little effect on markers of breast cancer risk. Furthermore, a pooled analysis that involved 9,514 breast cancer survivors found higher isoflavone intake was associated with a statistically significant 25% reduction in recurrence over the average 7.4-year follow-up period. Given the clinical and epidemiologic data, our position is that clinicians should allow soyfood use by patients for whom soyfoods already represent a normal part of their diet, and should not discourage other breast cancer survivors from moderate consumption.
Introduction

The relationship between soyfood intake and breast cancer has been rigorously investigated for 2 decades. The presence of isoflavones in soyfoods, diphenolic molecules classified as phytoestrogens, primarily accounts for the interest in this relationship. Initial focus was on the possible breast cancer–protective properties of soyfoods; a result of the (1) low historical breast cancer incidence rates in soyfood-
consuming countries, (2) increased breast cancer incidence rates among Japanese immigrants to the United States, and (3) chemopreventive properties of isoflavones observed in vitro.[1] Compelling yet speculative evidence suggests that soyfoods reduce breast cancer risk, but to derive this proposed benefit requires consumption during childhood and/or adolescence.[2]
In recent years attention has turned to the possible role of isoflavones in stimulating the growth of estrogen-sensitive breast tumors, as has been demonstrated in a widely used ovariectomized athymic mouse model.[3] As a result, clinicians generally advise breast cancer patients against regular soyfood consumption. A Canadian survey found that 25% of estrogen receptor (ER)-positive breast cancer patients stopped consuming soyfoods after receiving their diagnosis.[4] This issue is of obvious public health importance. In the United States alone, of the estimated 13.7 million Americans currently alive with a history of cancer, 41% had received a diagnosis of breast cancer.[5]
However, recently published clinical and prospective epidemiologic research questions the need for the advised restriction against soy. Also, doubts have been raised about the utility of the ovariectomized athymic mouse model for understanding the impact of isoflavones in humans. Furthermore, findings from the Women’s Health Initiative trials illustrate the uncertainty surrounding even the long-term use of estrogen,[6] as opposed to combination hormone therapy,[7] in the etiology of breast cancer. It is argued here that it is now time for oncologists and other clinicians to reconsider proscription against the use of soyfoods by women with a history of breast cancer.
Isoflavones
Among commonly consumed foods, only soybean-derived products provide physiologically relevant amounts of isoflavones. The soybean contains 12 different isoflavone isomers: the three aglycones genistein (4',5,7-trihydroxyisoflavone), daidzein (4',7-dihydroxyisoflavone), and glycitein (7,4'-dihydroxy-6-methoxyisoflavone); their respective β-glycosides genistin, daidzin, and glycitin; and three β-glucosides each esterified with either malonic or acetic acid.[8] (Isoflavone amounts used in this text refer to the aglycone equivalent weights.) When all forms of the individual isoflavones are considered, genistein, daidzein, and glycitein account for approximately 50%, 40%, and 10%, respectively, of the total soybean isoflavone content.[8]
There are approximately 3.5 mg of isoflavones per gram of protein in traditional soyfoods,[9] whereas in some more modern forms of soy as much as 80% of the isoflavone content is lost during processing. On average, there are 20–30 mg of isoflavones per serving of traditional soyfoods (eg, 250 mL soymilk, 100 g tofu), and older Japanese and Shanghai Chinese individuals consume about 30–50 mg of isoflavones per day.[9]
Isoflavones have a chemical structure similar to estrogens, bind to ERs, and exert estrogen-like effects under certain experimental conditions. For these reasons, isoflavones have been classified as phytoestrogens despite their many other biological mechanisms of action.[1] Genistein, which is the main circulating and best-studied isoflavone, transactivates ERα and induces estrogenic effects with 103–104 less potency than 17β-estradiol.[10] However, serum isoflavone concentrations following a high-soy meal can reach low micromolar levels,[11] thereby exceeding postmenopausal total estrogen levels by ~103.
In addition to being phytoestrogens, isoflavones are classified as selective ER modulators (SERMs).[12] The effect of any given SERM on a specific tissue depends upon the conformational shape of the ligand-receptor complex, the ERα:ERβ ratio, and the types of co-activators and co-repressors in cells. The tissue selectivity of isoflavones likely derives from their preferential binding to and transactivation of ERβ in comparison with ERα.[12] These two receptors have different tissue distributions and can have different functions. In the breast, activation of ERβ appears to inhibit the stimulatory and proliferative effects of ERα activation.[13]
The physiological implications of the preferential binding of genistein that occurs at the molecular level at certain concentrations are not fully understood. Nevertheless, there are numerous clinical examples of isoflavones exerting effects similar to those of estrogen on some tissues[14] without effects on other estrogen-sensitive endpoints,[15] although there is very limited evidence demonstrating anti-estrogenic effects.[16-18]
Finally, as mentioned previously, isoflavones potentially exert physiological effects independent of ER binding as they, especially genistein, affect signal transduction pathways in vitro by inhibiting the activity of enzymes (eg, tyrosine protein kinase, mitogen-activated kinase, DNA topoisomerase, etc.) and regulating cellular factors that control the growth and differentiation of cells.[1] The physiological relevance of many of these properties is unclear because the in vitro concentrations at which these effects are observed are generally far higher than can be achieved in vivo. Nevertheless, there is animal[19] and clinical[20] evidence of ER-independent effects of isoflavones.
Preclinical Effects of Genistein
Genistein exerts a biphasic effect on MCF-7 (an ER+ human breast cancer cell line) cell growth in vitro. At physiologically relevant concentrations, the estrogenic actions of genistein result in cell growth (in an estrogen-depleted media), whereas only at much higher concentrations do the ER-independent effects of genistein on signal transduction result in growth inhibition.[3] Because the estrogenic effects of genistein are minimized in an estrogen-rich medium/environment, it has been proposed that isoflavones function as estrogen antagonists in premenopausal women and as estrogen agonists in postmenopausal women. However, this conceptual framework is probably not applicable to breast tissue. Although circulating estrogen levels in postmenopausal women are only one-third those of premenopausal women, breast tissue estrogen concentrations are similar in women regardless of their menopausal status[21,22] because of local estrogen synthesis and uptake from the circulation.[23]
The in vitro stimulatory effects of genistein on MCF-7 cells were largely overlooked until this isoflavone was also found to stimulate the growth of mammary tumors in ovariectomized athymic mice implanted with these cells. It was subsequently shown that neither daidzein nor its metabolite equol has such effects[24] and genistein is without effect on ER-negative breast cancer cells.[25] In the basic model demonstrating stimulation, after tumors reach a cross-sectional area of ~30–40 mm2, the estrogen pellet, which is implanted to stimulate tumor growth, is removed, resulting in tumor regression in mice consuming a soy-free diet.[3] In contrast, after an initial period of regression, tumor regrowth occurs in mice fed a soy-free diet that is supplemented with a variety of genistein/genistin-containing products.[3]
Research from the above-described model also shows that genistein inhibits the efficacy of tamoxifen[26] and letrozole,[27] and that despite containing similar amounts of genistein/genistin, tumor growth is positively related to the degree to which the isoflavone-containing product has been processed.[28] For example, soy flour (the least processed soyfood evaluated) does not stimulate tumor growth (although it does not allow regression to occur), whereas isolated genistein stimulates tumors to a greater extent than mixed isoflavones.[28] This processing effect has helped garner support for the use of minimally processed soyfoods by breast cancer patients but has also led to strong warnings against the use of soy (isoflavone) supplements. However, this distinction among different genistein-containing products has not been tested clinically. Such investigations are warranted given that recent evidence indicates that the “processing effect” is not applicable to humans.[29]
Clinical Research Involving Markers of Breast Cancer Risk
TABLE 1
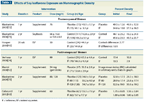
Effects of Soy Isoflavone Exposure on Mammographic Density
Numerous studies have evaluated the effects of isoflavone exposure on markers of breast cancer risk, including reproductive hormone levels, mammographic density, nipple aspirate fluid contents, and cell proliferation (Tables 1 and 2).[30,31] Agents that raise breast cancer risk, eg, combined hormone therapy, increase mammographic density[32] and cell proliferation,[33] whereas agents that lower risk, such as tamoxifen, decrease these markers.[34] As discussed below, the totality of evidence indicates that regardless of the source, isoflavone exposure does not adversely affect breast tissue.
TABLE 2

Description of Studies Evaluating Effects of Soy Isoflavone Exposure on Breast Cell Proliferation
Reproductive hormones
In a meta-analysis by Hooper et al[35] that included 35 studies involving postmenopausal women, isoflavones had no statistically significant effects on estradiol, estrone, sex hormone binding globulin (SHBG), follicle-stimulating hormone (FSH), or luteinizing hormone (LH), although there was a small statistically nonsignificant increase (~14%) in total estradiol. While acknowledging that higher circulating estradiol levels are related to a higher breast cancer risk,[36] Huber et al[37] pointed out that in the meta-analysis,[35] a parallel decrease in estrone levels occurred in response to soy/isoflavones. Elevated estrone levels are associated with a similar increase in breast cancer risk among postmenopausal women.[36] Thus, the opposing small and nonsignificant effects on hormone levels are likely of no relevance to breast cancer risk.
In the 11 studies involving premenopausal women in the meta-analysis, soy or isoflavone consumption did not affect primary outcomes-estradiol, estrone, or SHBG concentrations, but it significantly reduced secondary outcomes-FSH (~ 22%, P = .01) and LH (~24%, P = .05), and menstrual cycle length was increased by 1.05 days (95% confidence interval [CI]: 0.13, 1.97; 10 studies).[35] The authors were unable to conclude whether the changes in FSH and LH reflect an estrogenic or antiestrogenic effect,[35] and another group of investigators has noted that longer menstrual cycles are associated with a reduced breast cancer risk.[38]
Mammographic density
Greater breast tissue density as seen in radiographic images is associated with a higher breast cancer risk after adjusting for age, parity, and body weight.[39] Although the effects of estrogen-only treatment vs estrogen plus progestin on density are quite modest, they have been observed across studies. For example, in the Postmenopausal Estrogen/Progestin Interventions (PEPI) randomized placebo-controlled clinical trial, conjugated equine estrogen (CEE) treatment resulted in a nonsignificant increase in mammographic density of 1.2% after 1 year compared with significant increases of 3.1% to 4.8% for different estrogen plus progestin regimens.[40]
In a meta-analysis by Hooper et al,[41] isoflavone exposure did not affect mammographic density in postmenopausal women (see Table 1 for a description of studies). Two other studies not included in the meta-analysis support this conclusion,[42,43] and a third shows isoflavone exposure does not lead to abnormal mammograms (cancer, cysts, or other abnormalities).[44] In premenopausal women, isoflavone exposure modestly increased breast density by 1.83%, but due to the small effect size the authors concluded that there were no immediate implications for practice (Table 1).[41]
Nipple aspirate fluid (NAF)
In nonlactating women, nipple aspiration is a noninvasive method to obtain breast fluid and epithelial cells, and it provides information on cellular and noncellular markers of breast cancer risk. Women who produce NAF appear to be at higher risk of developing breast cancer.[45] The first study to investigate the effects of soy on NAF was a single-arm intervention among 24 pre- and postmenopausal Caucasian women that intervened with 38 grams daily of isolated soy protein.[46] Each woman served as her own control and donated NAF monthly for 6 months of soy supplementation and 3 months preceding and following the active intervention. In the 14 premenopausal women, the volume of NAF increased two- to six-fold as compared to baseline during the intervention: 11.5, 18.2, 28.4, and 32.6 mL for months 1–3, months 4–6, months 7–9, and months 10–12, respectively (P = .03). The presence of hyperplastic epithelial cells in the NAF in four women during the months of soy protein intake raised concern that NAF secretors may be sensitive to soy and react with increased secretion and cell proliferation.
However, later investigations did not confirm that suggestion.[47-49] The largest study, a randomized cross-over study conducted in Hawaii, randomized 96 women to a high-soy diet or a low-soy diet for 6 months, without observing significant changes in estrogen levels in NAF and serum.[49] In another study, there were no treatment effects on any NAF parameters in either pre- or postmenopausal women after high-dose isoflavone (239 mg/d) supplementation for 6 months.[48]
Breast cell proliferation
Epithelial cell proliferation serves as an important prognostic marker in human breast cancer[50] and may help predict risk associated with different hormonal agents.[51] A common method for evaluating proliferation is the immunohistochemical marker Ki67 (also called MIB1), which is a nuclear protein expressed by cells in all active phases of the cell cycle but not in quiescent or resting cells.[52] Ki67 labeling correlates significantly with higher carcinoma grade, clinical response to endocrine therapy, higher risk of relapse, and worse survival in patients with early breast cancer.[30]
Five studies evaluated the effects of soy-derived isoflavones on breast cell proliferation (Table 2). The intervention period ranged from 14 days[53] to 1 year,[54] and the isoflavone dose ranged from 36 mg/d[55] to 239 mg/d.[48] All but one study used isoflavone supplements.[53] Two studies involved postmenopausal women only,[54,55] one involved mostly postmenopausal women,[56] one focused on premenopausal women only,[53] and one was equally divided between pre- and postmenopausal women.[48] None of the studies found an increase in cell proliferation in response to isoflavones relative to the placebo. One study did find cell proliferation increased relative to baseline in premenopausal women (Table 2) (1.71 to 2.18, P = .04).[48] This finding has led to cautionary statements about the use of supplements, and to a lesser extent, soyfoods.[57] However, our view is that this study provides strong evidence that isoflavone exposure consistent with intake levels among women in Asian countries is safe.
Of the total daily 235-mg dose of isoflavones used in this study, 150 mg were from genistein, the isoflavone shown to stimulate tumors in ovariectomized athymic mice.[58] This much genistein is provided by approximately 15 servings of soyfoods. And yet, neither cell proliferation nor atypia were increased in postmenopausal women, the group presumed to be most vulnerable to the proposed breast tissue-proliferative effects of isoflavones.
TABLE 3
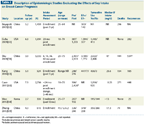
Description of Epidemiologic Studies Evaluating the Effects of Soy Intake on Breast Cancer Prognosis
Epidemiologic Research in Breast Cancer Survivors
Seven prospective epidemiologic studies have evaluated the impact of post-diagnosis soy intake on the prognosis of breast cancer survivors (Table 3).[59-65] The largest investigations with the longest follow-up periods are the Shanghai Breast Cancer Survival Study (SBCSS),[59] the Women’s Healthy Eating and Living (WHEL) study,[60] and the Life After Cancer Epidemiology (LACE) study.[61] The Chinese investigation found that when comparing the highest soy protein intake quartile (> 15.72 g/d) to the lowest, total mortality (hazard ratio [HR] = 0.71; 95% CI, 0.54–0.92) and recurrence (HR = 0.68; 95% CI, 0.54–0.87) were reduced by approximately 30%.[59]
The relevance of these striking findings to non-Chinese women has been questioned because of the possibility that tumors that develop in Chinese women who consumed soy early in life respond differently to isoflavones than tumors in non-Chinese women who have not been previously exposed to soy.[66] While this question cannot be definitively answered by the current studies, protective effects of soy consumption were also found in the LACE[61] and WHEL[60] studies, which comprised predominantly Caucasian women who were unlikely to have consumed soy when young. As expected, isoflavone intake in the US studies was very low, raising concerns about the utility of the data. However, because a 95th percentile isoflavone intake category was created (women in this category consumed the equivalent of about one serving of a traditional soyfood per day), both US studies were able to examine soy intake at levels comparable to those of women living in Asian countries.
Recently, Nechuta et al[67] pooled results from the SBCSS and the WHEL and LACE studies. The 9,514 breast cancer patients in this analysis were followed for a mean of 7.4 years. When comparing the highest isoflavone intake group with the lowest (≥ 10 mg/d vs < 4 mg/d), risk of total mortality, breast cancer–specific mortality, and breast cancer recurrence were reduced by 13% (HR = 0.87; 95% CI, 0.70–1.10), 17% (HR = 0.83; 95% CI, 0.64–1.07), and 25% (HR = 0.75; 95% CI, 0.61–0.92), respectively. Soy consumption tended to reduce recurrence to a greater extent in tamoxifen users than in non-users (HR = 0.63 vs 0.79), ER-negative vs ER-positive patients (HR = 0.64 vs 0.81), and postmenopausal vs premenopausal women (HR = 0.64 vs 0.93), although there were no statistically significant interactions observed among these strata.
The suggestion that soy intake was more beneficial in women with a history of tamoxifen use is especially notable because the opposite would have been predicted based on the results from the ovariectomized athymic mouse model discussed previously.[26] Interestingly, in the small study by Kang et al,[63] (Table 3) not only did soy intake not affect the efficacy of tamoxifen, it enhanced the efficacy of anastrozole, another finding which contrasts with that from the ovariectomized athymic mouse model.[27]
Finally, there is a need for better understanding of the impact of soy and isoflavone exposure on the prognosis of survivors according to the different biological subtypes of breast cancer, such as those expressing human epidermal growth factor receptor-2 (HER2). The research that exists specifically with respect to tumors expressing HER2 is far too limited to use even as a basis for speculation.[64,68]
Summary
The evidence to date convincingly suggests that soyfoods are not harmful to breast cancer survivors. In fact, the prospective epidemiologic data suggest benefit from consuming soy after a diagnosis of breast cancer. It is noteworthy that soy intake is associated with improved prognosis in epidemiologic studies, whereas intervention studies indicate no effect of isoflavone exposure on breast tissue. It is therefore possible that soyfoods have effects that differ from the isoflavone products used in the interventions and/or that breast cancer prognosis is improved through mechanisms not detected by changes in cell proliferation or breast tissue density, such as by angiogenesis inhibition.[69]
The totality of the reviewed evidence provides no basis for advising breast cancer patients, especially postmenopausal women, against the consumption of soyfoods. However, since the epidemiologic data, but not the clinical data, are supportive of potential benefits, sufficient evidence to recommend soyfoods to breast cancer patients to improve prognosis does not exist at this time. Therefore, the most justifiable position for clinicians is to allow soyfood use by patients for whom soyfoods already represent a normal part of their diet (mainly vegetarians and patients of Asian ethnicity) and not to discourage other survivors from moderate consumption. This recommendation is consistent with the position of the American Cancer Society[70] and the American Institute for Cancer Research.[71]
Finally, we acknowledge that it would be easy for clinicians to advise patients against the use of soyfoods, especially in light of the lack of definitive clinical data, to avoid any potential adverse effects on prognosis. However, this decision may deprive women of a food that has nutritional benefits, especially with respect to cardiovascular disease. Breast cancer patients need to focus on an overall healthy lifestyle, which includes diet, because substantial numbers will live for many years without recurrence and therefore are at risk for other chronic diseases related to aging. Given the lack of human evidence for adverse effects, soyfoods can be part of a healthy lifestyle for breast cancer patients and survivors.
Financial Disclosure:Dr. Messina is an advisor to the United Soybean Board, and an advisor/consultant to Archer Daniels Midland Company and White Wave Foods. The remaining authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744-57.
2. Messina M, Wu AH. Perspectives on the soy-breast cancer relation. Am J Clin Nutr. 2009;89:1673S-1679S.
3. Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;16:219-26.
4. Boucher BA, Cotterchio M, Curca IA, et al. Intake of phytoestrogen foods and supplements among women recently diagnosed with breast cancer in Ontario, Canada. Nutr Cancer. 2012;64:695-703.
5. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220-41.
6. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-86.
7. Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010; 304:1684-92.
8. Murphy PA, Barua K, Hauck CC. Solvent extraction selection in the determination of isoflavones in soy foods. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:129-38.
9. Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1-12.
10. Muthyala RS, Ju YH, Sheng S, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559-67.
11. Mathey J, Lamothe V, Coxam V, et al. Concentrations of isoflavones in plasma and urine of post-menopausal women chronically ingesting high quantities of soy isoflavones. J Pharm Biomed Anal. 2006;41:957-65.
12. Oseni T, Patel R, Pyle J, et al. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008;74:1656-65.
13. Speirs V, Carder PJ, Lane S, et al. Oestrogen receptor beta: what it means for patients with breast cancer. Lancet Oncol. 2004;5:174-81.
14. Taku K, Melby MK, Kronenberg F, et al. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: systematic review and meta-analysis of randomized controlled trials. Menopause. 2012;19:776-90.
15. Alekel DL, Van Loan MD, Koehler KJ, et al. The soy isoflavones for reducing bone loss (SIRBL) study: a 3-y randomized controlled trial in postmenopausal women. Am J Clin Nutr. 2010;91:218-30.
16. Sampey BP, Lewis TD, Barbier CS, et al. Genistein effects on stromal cells determines epithelial proliferation in endometrial co-cultures. Exp Mol Pathol. 2011;90:257-63.
17. Murray MJ, Meyer WR, Lessey BA, et al. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: a pilot trial. Menopause. 2003;10:456-64.
18. Kayisli UA, Aksu CA, Berkkanoglu M, et al. Estrogenicity of isoflavones on human endometrial stromal and glandular cells. J Clin Endocrinol Metab. 2002;87:5539-44.
19. Zhou JR, Yu L, Zhong Y, et al. Inhibition of orthotopic growth and metastasis of androgen-sensitive human prostate tumors in mice by bioactive soybean components. Prostate. 2002;53:143-53.
20. Lazarevic B, Hammarstrom C, Yang J, et al. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br J Nutr. 2012:1-10.
21. Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol. 2003;86:245-53.
22. Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225-30.
23. Haynes BP, Straume AH, Geisler J, et al. Intratumoral estrogen disposition in breast cancer. Clin Cancer Res. 2010;16:1790-801.
24. Ju YH, Fultz J, Allred KF, et al. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856-63.
25. Santell RC, Kieu N, Helferich WG. Genistein inhibits growth of estrogen-independent human breast cancer cells in culture but not in athymic mice. J Nutr. 2000;130:1665-9.
26. Du M, Yang X, Hartman JA, et al. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis. 2012;33:895-901.
27. Ju YH, Doerge DR, Woodling KA, et al. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008;29:2162-8.
28. Allred CD, Allred KF, Ju YH, et al. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis. 2004;25:1649-57.
29. Setchell KD, Brown NM, Zhao X, et al. Soy isoflavone phase II metabolism differs between rodents and humans: implications for the effect on breast cancer risk. Am J Clin Nutr. 2011;94:1284-94.
30. de Azambuja E, Cardoso F, de Castro G, Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504-13.
31. DeCensi A, Guerrieri-Gonzaga A, Gandini S, et al. Prognostic significance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol. 2011;22:582-7.
32. Lundstrom E, Hirschberg AL, Soderqvist G. Digitized assessment of mammographic breast density--effects of continuous combined hormone therapy, tibolone and black cohosh compared to placebo. Maturitas. 2011;70:361-4.
33. Conner P. Breast response to menopausal hormone therapy--aspects on proliferation, apoptosis and mammographic density. Ann Med. 2007;39:28-41.
34. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101:384-98.
35. Hooper L, Ryder JJ, Kurzer MS, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423-40.
36. Key T, Appleby P, Barnes I, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606-16.
37. Huber J, Imhof M, Schmidt M. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:110-11; author reply 111-12.
38. Olsson H, Landin-Olsson M, Gullberg B. Retrospective assessment of menstrual cycle length in patients with breast cancer, in patients with benign breast disease, and in women without breast disease. J Natl Cancer Inst. 1983;70:17-20.
39. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-69.
40. Greendale GA, Reboussin BA, Slone S, et al. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95: 30-7.
41. Hooper L, Madhavan G, Tice JA, et al. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2010;16:745-60.
42. Marini H, Bitto A, Altavilla D, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. 2008;93:4787-96.
43. Colacurci N, De Franciscis P, Atlante M, et al. Endometrial, breast and liver safety of soy isoflavones plus Lactobacillus sporogenes in post-menopausal women. Gynecol Endocrinol. 2013;29:209-12.
44. Chilibeck PD, Vatanparast H, Pierson R, et al. Effect of exercise training combined with isoflavone supplementation on bone and lipids in postmenopausal women: a randomized clinical trial. J Bone Miner Res. 2013;28:780-93.
45. Wrensch MR, Petrakis NL, Miike R, et al. Breast cancer risk in women with abnormal cytology in nipple aspirates of breast fluid. J Natl Cancer Inst. 2001;93:1791-8.
46. Petrakis NL, Barnes S, King EB, et al. Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785-94.
47. Maskarinec G, Hebshi S, Custer L, et al. The relation of soy intake and isoflavone levels in nipple aspirate fluid. Eur J Cancer Prev. 2008;17:67-70.
48. Khan SA, Chatterton RT, Michel N, et al. Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer Prev Res (Phila). 2012;5:309-19.
49. Maskarinec G, Ollberding NJ, Conroy SM, et al. Estrogen levels in nipple aspirate fluid and serum during a randomized soy trial. Cancer Epidemiol Biomarkers Prev. 2011;20:1815-21.
50. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212-20.
51. Hofseth LJ, Raafat AM, Osuch JR, et al. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84:4559-65.
52. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-22.
53. Hargreaves DF, Potten CS, Harding C, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017-24.
54. Palomares MR, Hopper L, Goldstein L, et al. Effect of soy isoflavones on breast proliferation in postmenopausal breast cancer survivors. Breast Cancer Res Treat. 2004;88(Suppl 1):Abstr 4002.
55. Cheng G, Wilczek B, Warner M, et al. Isoflavone treatment for acute menopausal symptoms. Menopause. 2007;14:468-73.
56. Sartippour MR, Rao JY, Apple S, et al. A pilot clinical study of short-term isoflavone supplements in breast cancer patients. Nutr Cancer. 2004;49:59-65.
57. Khan SA, Chatterton RT, Bergan R. Soy isoflavones for breast cancer risk reduction-response. Cancer Prev Res. 2012;5:986-7.
58. Ju YH, Allred CD, Allred KF, et al. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957-62.
59. Shu XO, Zheng Y, Cai H, et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437-43.
60. Caan BJ, Natarajan L, Parker B, et al. Soy food consumption and breast cancer prognosis. Cancer Epidemiol Biomarkers Prev. 2011;20:854-8.
61. Guha N, Kwan ML, Quesenberry CP, Jr, et al. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. 2009;118:395-405.
62. Boyapati SM, Shu XO, Ruan ZX, et al. Soyfood intake and breast cancer survival: a followup of the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2005;92:11-7.
63. Kang X, Zhang Q, Wang S, et al. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ. 2010;182:1857-62.
64. Woo HD, Park KS, Ro J, et al. Differential influence of dietary soy intake on the risk of breast cancer recurrence related to HER2 status. Nutr Cancer. 2012;64:198-205.
65. Zhang YF, Kang HB, Li BL, et al. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac J Cancer Prev. 2012;13:479-82.
66. Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr. 2010;140:2326S-2334S.
67. Nechuta SJ, Caan BJ, Chen WY, et al. Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr. 2012;96:123-32.
68. Yang X, Yang S, McKimmey C, et al. Genistein induces enhanced growth promotion in ER-positive/erbB-2-overexpressing breast cancers by ER-erbB-2 cross talk and p27/kip1 downregulation. Carcinogenesis. 2010;31:695-702.
69. Kang X, Jin S, Zhang Q. Antitumor and antiangiogenic activity of soy phytoestrogen on 7,12-dimethylbenz[alpha]anthracene-induced mammary tumors following ovariectomy in Sprague-Dawley rats. J Food Sci. 2009;74:H237-42.
70. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:242-74.
71. American Institute for Cancer Research. Soy is safe for breast cancer survivors. 2012. Available from: http://www.aicr.org/cancer-research-update/november_21_2012/cru-soy-safe.html.
72. Maskarinec G, Williams AE, Carlin L. Mammographic densities in a one-year isoflavone intervention. Eur J Cancer Prev. 2003;12:165-9.
73. Maskarinec G, Takata Y, Franke AA, et al. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134:3089-94.
74. Verheus M, van Gils CH, Kreijkamp-Kaspers S, et al. Soy protein containing isoflavones and mammographic density in a randomized controlled trial in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2632-8.
75. Maskarinec G, Verheus M, Steinberg FM, et al. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr. 2009;139:981-6.