Integrative Oncology in Young Women With Breast Cancer
Yancey Warren, Jr, MD, MAT, and colleagues investigate the use of integrative oncology services among young women with breast cancer.
ABSTRACT
Small studies have demonstrated the benefit of integrative oncology (IO) therapies in patients with breast cancer; however, referral patterns and timing of therapies are unknown. This study describes the referral pattern and utilization of IO services by young women with breast cancer.
A retrospective review identified female patients, 40 years or younger, with a breast cancer diagnosis between 2014 and 2019, and a documented IO consultation. Patient demographics, cancer characteristics, treatments, reasons for seeking and timing of IO consultation, and IO treatment modalities were analyzed. The IO program treated 64 young women with a median age of 38.6 years. Clinical staging was primarily IA (27%), IIA (34%), or IIB (27%), and 64% of patients were clinically node negative with no evidence of metastasis. Women utilized the IO program for recurrence risk reduction and for treatment-related adverse effects (TRAEs), most commonly vasomotor complaints (44%). Therapies utilized were acupuncture (36%), healing touch (28%), oncology massage (30%), and other (75%; music therapy, therapeutic art, spiritual care, meditation, t’ai chi, yoga, and nutrition), which were commonly initiated during treatment (69%).
Our data suggest that young women utilize IO services to reduce their future cancer risk and TRAEs, but they are often referred after standard cancer care treatments have begun. Future studies could examine the optimal timing for IO intervention.
KEY WORDS
Integrative medicine, integrative oncology, complementary medicine, alternative medicine, breast cancer, referral pattern, young women
Oncology (Williston Park). 2022;36(11):658-663.
DOI: 10.46883/2022.25920978
Introduction
Of the 1.3 million new cases of breast cancer diagnosed annually worldwide, approximately 7% are diagnosed in young women, defined as 40 years and younger. With such a high incidence worldwide, this translates to approximately 91,000 new cases of breast cancer annually in young women. Of the overall new annual diagnoses, 2.5% (32,500) will be in women younger than 35 years, and approximately 1% (13,000) will be in women younger than 30 years. Levine Cancer Institute in Charlotte, North Carolina, will treat approximately 90 to 100 of these young women each year. This represents a significant number of women with a breast cancer diagnosis who have a unique set of needs and challenges to overcome throughout their treatment.1-3
Compared with women older than 40 years, young women with breast cancer tend to present with larger, higher-grade tumors that are more likely to be hormone receptor–negative and carry a worse prognosis. These women have a higher likelihood of nodal involvement and more advanced stage at time of diagnosis. Additionally, in the long term, these women, compared with older women with breast cancer, are at increased risk of recurrence (based on Oncotype Dx Recurrence Scores) and increased risk of cancer-related death.4-7 These risk factors often result in more aggressive treatments, placing patients at a higher risk of treatment-related adverse effects (TRAEs) and toxicities such as fertility issues, vasomotor symptoms, amenorrhea, decreased bone density, early menopause, sexual dysfunction, and cognitive AEs.4,8-12 These AEs can lead to more treatment nonadherence in younger vs older women.13,14 All of the aforementioned factors add to the complexity of treating young women with breast cancer. Further, this scenario calls forth the importance and necessity of a multidisciplinary, person-centered treatment approach.15
The use of integrative oncology (IO) as an adjunct to standard cancer treatment may help to reduce TRAEs, improve treatment compliance, and enhance patient well-being.16,17 As defined by the Society for Integrative Oncology (SIO), IO is “a patient-centered, evidence-informed field of cancer care that utilizes mind and body practices, natural products, and/or lifestyle modifications from different traditions alongside conventional cancer treatments. It aims to optimize health, quality of life, and clinical outcomes across the cancer care continuum and to empower people to prevent cancer and become active participants before, during, and beyond cancer treatment.”18 Small studies in patients with breast cancer suggest that integrative therapies may be of benefit;18 however, the types and timing of integrative therapies offered at cancer centers differ. Furthermore, the timing of integrative therapies and usage patterns relative to standard treatment are unknown.
In 2013, our large cancer care center established an IO section within the Department of Supportive Oncology and an IO clinic was created. This clinic houses fellowship-trained IO physicians and an advanced practice provider. Any provider on the multidisciplinary team may refer a patient for IO consultation based on patient symptomology or patient request. As a part of the IO clinic, patients are offered comprehensive services as adjunctive treatments to standard of care (SOC). These services include individual modalities such as acupuncture, healing touch and oncology massage, music therapy, therapeutic art, and spiritual care as well as group classes such as meditation, t’ai chi, yoga, nutrition, and dietary supplementation. Additionally, in January 2018 the Sandra Levine Young Women’s Breast Cancer Program (YWBP) was established at our cancer center to enhance the care given to young women with breast cancer.
The aim of this study was to learn from the established patterns of use of IO therapies in our patient population of young women with breast cancer to better establish referral practice patterns for our institution and to further define optimal timing of these therapies for this specific population. We sought to identify which integrative therapies our young patients used throughout their treatment course and the timing of each therapy used. The primary outcome of this study was to identify which IO modalities were used by young patients with breast cancer. The secondary outcome was to recognize the timing of when these modalities were used.
Methods
This study was approved by the Atrium Health Institutional Review Board. A retrospective, electronic medical record review was performed to identify women 40 years or younger who got a diagnosis of breast cancer from January 1, 2014, through December 31, 2019. Based on the study exclusion criteria, all patients who were male, older than 40 years, did not have a breast cancer diagnosis, or did not have documented IO consultation were excluded. All female patients with a breast cancer diagnosis, 40 years or younger, with a documented IO consultation were included in the study. Demographics (age, body mass index [BMI], race, insurance status), tumor characteristics (size, histology, hormone receptor status), nodal status, clinical and pathologic stage, breast surgery performed (none vs lumpectomy vs mastectomy), nodal surgery performed (none vs sentinel node biopsy vs full axillary dissection), chemotherapy timing (none vs neoadjuvant vs adjuvant), and use of radiation (none vs adjuvant) were included for analysis. These data were analyzed by using descriptive statistics (counts, percentages, median, IQR, and χ2 statistics).
Timing of IO referral relative to chemotherapy (pre- vs mid- vs post chemotherapy) was determined. Reason for IO referral was divided into 2 categories: cancer recurrence risk reduction and TRAEs. Cancer recurrence risk reduction included lifestyle and dietary modifications associated with lowering an individual’s cancer risk.16,17 TRAEs included insomnia, pain, weight loss or gain, chemotherapy-induced peripheral neuropathy, anxiety, fertility issues, vasomotor complaints (hot flashes and rash), gastrointestinal complaints (chemotherapy-induced nausea and vomiting; nausea), and problems related to nutrition, memory, and mood. Integrative modalities used by patients were acupuncture, healing touch and oncology massage, and “other,” which includes music therapy, therapeutic art, and spiritual care as well as group classes such as meditation, t’ai chi, yoga, nutrition, and dietary supplementation. Timing of IO consultation, reason for referral, and modalities used by patients were analyzed by using descriptive statistics. All statistics were calculated using SAS 9.4 and significance was assessed at P <.05.
Results
TABLE 1. Patient Demographics and Characteristics
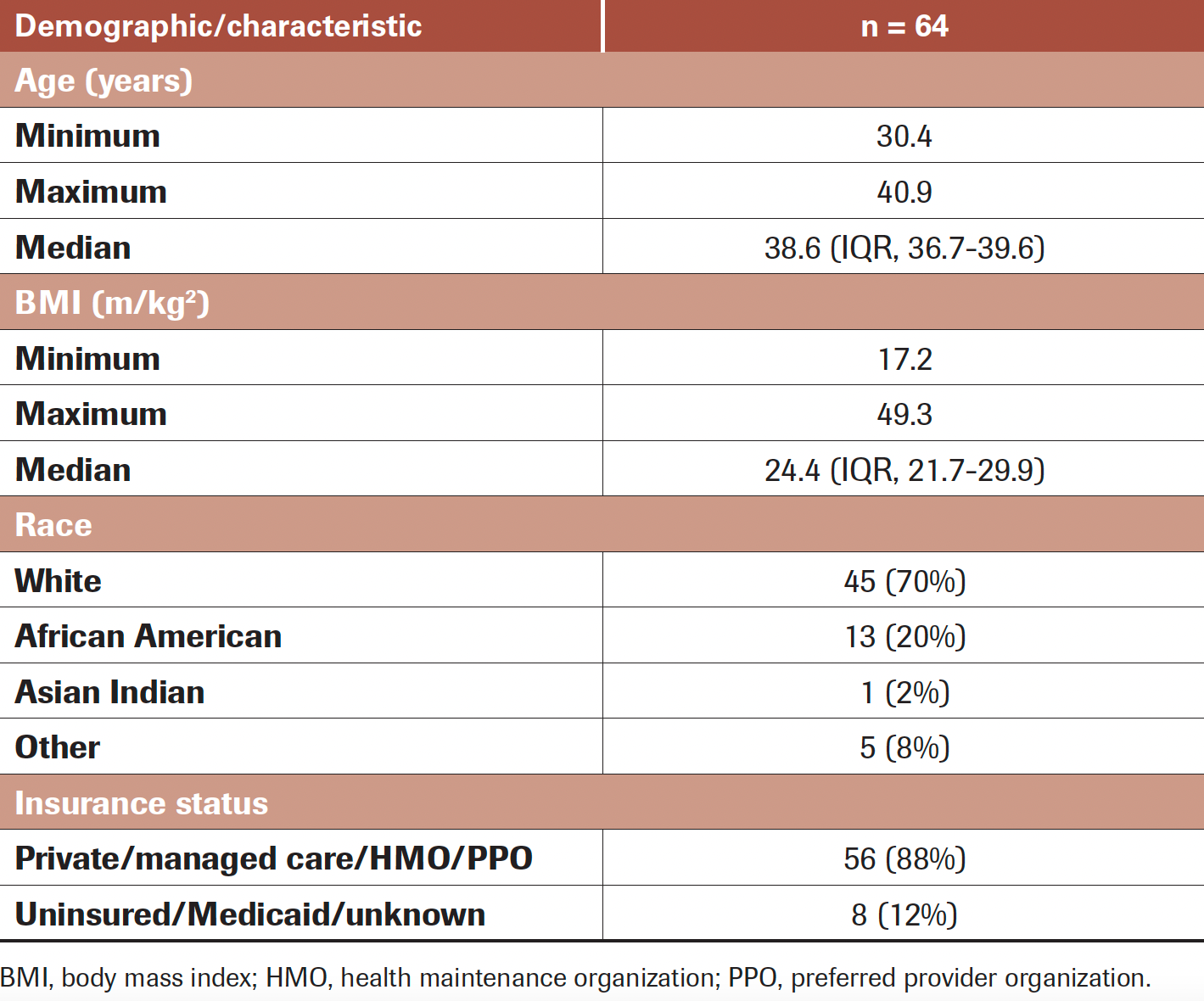
In our institutional cancer database, a total of 541 patients 40 years or younger got a diagnosis of breast cancer from 2014 to 2019. Of these young women, 64 (12%) sought out and participated in an IO consultation during the 5-year period. Table 1 displays demographic data regarding the patient population. Median age was 38.6 years (IQR, 36.7-39.6), and while the women in this clinic were 40 years or younger, the majority were older than 35 years (91%), and none were younger than 30 years. Median BMI was 24.4 m/kg2 (IQR, 21.7-29.9); however, we saw patients across the spectrum, from underweight to severely obese. Patients were primarily White (70%) or African American (20%).
TABLE 2. Cancer Characteristics of the Study Population
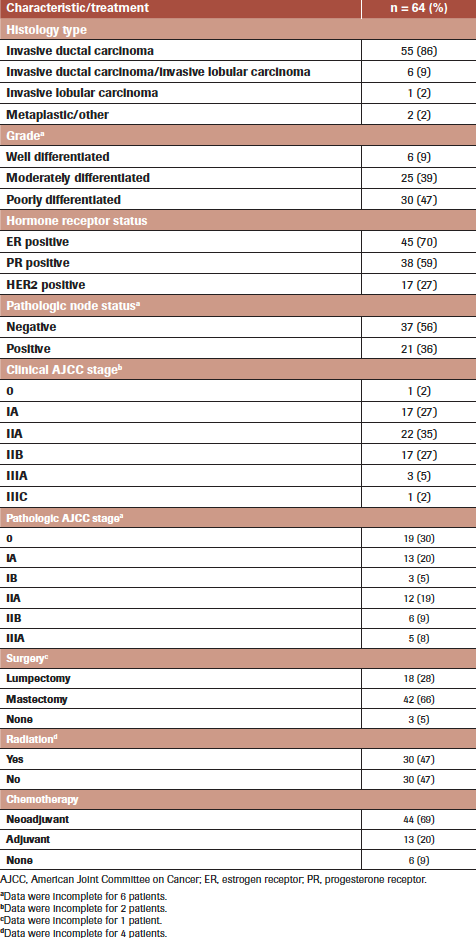
Table 2 displays cancer characteristics and treatment modalities of the 64 patients examined in the study. The most common patient baseline factors at presentation were hormone receptor–positive status (estrogen receptor, 70%; progesterone receptor, 59%), poorly differentiated grade (47%), and invasive ductal carcinoma histology (86%). Node-negative (64%) disease without distal metastases was common. Clinical stages IA, IIA, and IIB were most common and almost evenly distributed (27%, 34%, and 27%, respectively), whereas only 2% of patients were clinical stage 0. In general, the pathologic stage of patients was lower than that of their clinical stage, likely due to the effects of neoadjuvant treatment. On pathologic examination, 30% of patients were pathologic stage 0, 20% stage IA, and 19% stage IIA.
Table 2 documents treatment modalities. A total of 63 (98%) patients received chemotherapy; 44 (69%) received it in the neoadjuvant setting and 37 (58%) began chemotherapy treatment prior to their IO consultation. The mastectomy rate was 65%, while 29% underwent breast conservation surgery (BCS). Three (5%) patients did not undergo surgery: 1 had clinical stage IV disease at diagnosis, 1 progressed from stage IIB to IV while on treatment prior to surgery, and 1 elected to undergo treatment elsewhere. Of the patients who had surgery, 47% received adjuvant radiation therapy (18 [43%] patients with mastectomy, 11 [61%] with BCS). Data regarding the use of adjuvant radiation were unavailable for 2 patients with mastectomy. Of those who did not undergo surgery, 1 patient underwent radiation therapy, 1 did not, and data regarding the use of radiation were unavailable for the third.
TABLE 3. Reason for Seeking IO Consultation, Timing of IO Treatment, and Most Common IO Modalities Used
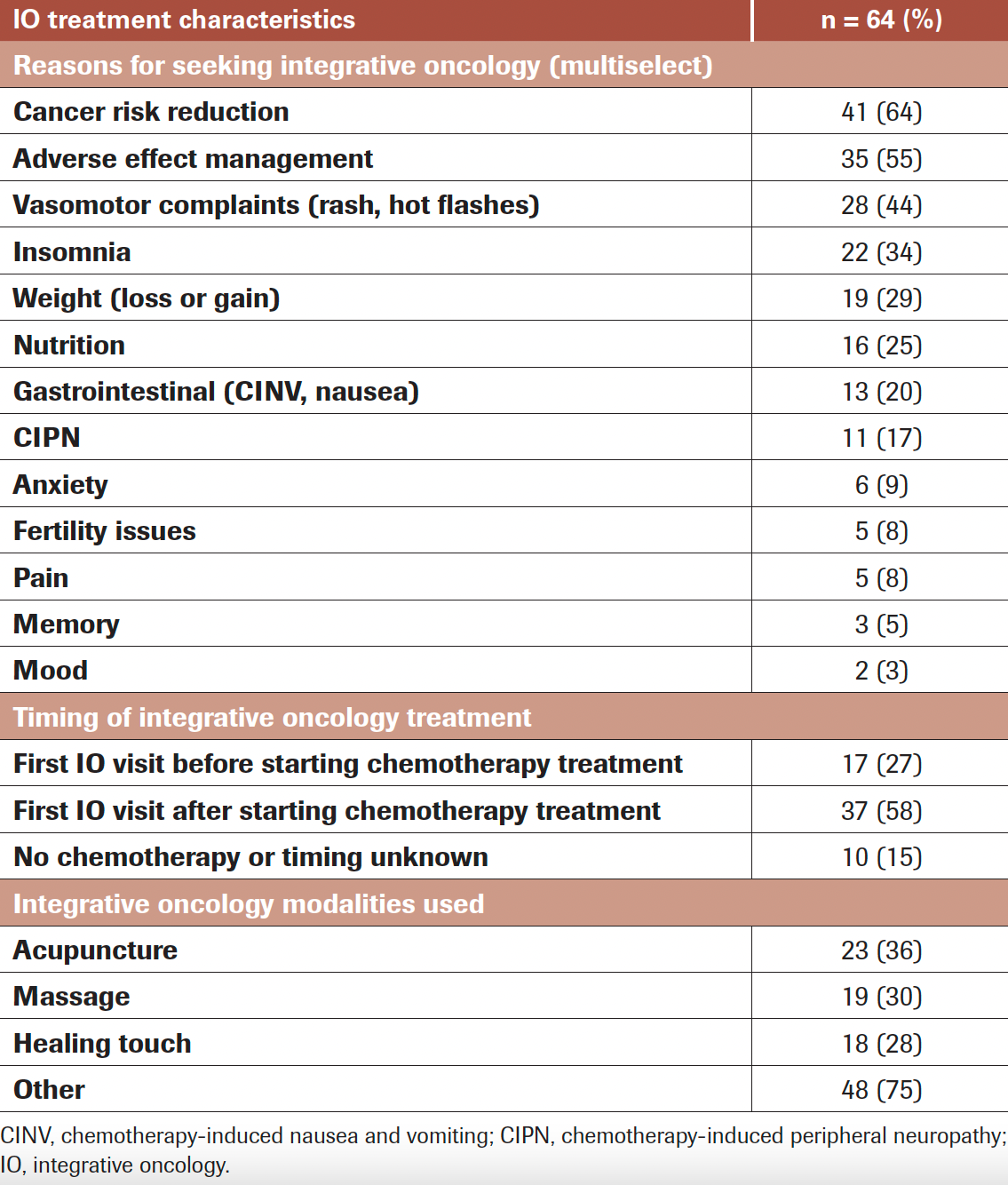
Table 3 provides data regarding the timing of IO consultation along with the reason for seeking consultation and which therapy modalities were utilized. Of the 54 young women with known chemotherapy start dates, 25% of patients were referred prior to initiating treatment and 75% were referred after treatment had already begun. Seventeen (31%) women participated in an IO consultation prior to initiating any standard treatment while 37 (69%) were seen after initiating therapy, which is significantly higher (P <.01) compared with the assumption that the distribution would be evenly split between before or after treatment initiation. The primary reasons women sought an IO consultation can be divided into 2 broad categories: cancer recurrence risk reduction (64%) and reduction of TRAEs (55%). For the latter, this was subdivided into patient-specific AEs, with the most common being vasomotor concerns (44%), such as hot flashes and other premenopausal symptoms. Other common AEs prompting IO consultation and treatment included insomnia (34%), weight gain/loss (30%), nutritional issues (25%), and gastrointestinal problems such as chemotherapy-induced nausea/vomiting (20%). Therapies recommended by IO specialists were relatively evenly distributed regardless of the AE that prompted visitation: acupuncture (36%), massage (30%), and healing touch (28%).
Discussion
Role of Integrative Oncology in Patients With Breast Cancer
Young women with breast cancer often have more aggressive cancers that portend poorer prognoses and require more aggressive treatments.4-7 These aggressive treatments place these patients, in particular, at a higher risk of AEs and, subsequently, a higher likelihood of treatment nonadherence or inability to complete treatment.4,8-14 IO promotes the utilization of comprehensive, evidence-based complementary therapies in an effort to improve the lives of patients undergoing SOC cancer treatments. Patients with breast cancer have historically utilized these therapies more than any other cancer patient population, thus most of the relevant, evidence-based IO research that is currently available comes from this patient population. For this reason, guidelines were developed jointly by the SIO and the American Society of Clinical Oncology based on a systematic review of the literature for evidence-based use of integrative therapies during and after breast cancer treatment.16 These guidelines outline the level of evidence for associated benefits and harms for each integrative modality in the oncology setting. Based on review of current SIO guidelines, as well as other data available in the literature, we have defined the role of IO therapies for multiple clinical applications. While the guidelines are not specific for young women with breast cancer, they can be broadly applied to this specific population as well.
Twelve percent of patients in our study population participated in an IO consultation. The most common reason young patients at our institution sought IO consultation was for vasomotor symptoms related to the use of antihormonal therapy (28%). These drugs are SOC for adjuvant systemic treatment for patients with hormone receptor–positive breast cancers because they dramatically decrease the risk of cancer recurrence; however, they are also frequently associated with the development of vasomotor symptoms (hot flashes, night sweats, heart palpitations, and anxiety).19,20 By far, hot flashes (43%) was the most common of these symptoms experienced by patients seen in our IO clinic. Due to its promising results in the treatment of hot flashes, acupuncture is offered to all patients presenting with hot flashes, and it was utilized by 23% of patients in our study.21-24
Weight fluctuations—both gain and loss—are common during cancer treatment. A total of 29% of patients seen in the IO clinic had concerns regarding their weight and 25% presented with nutritional concerns. Depending on their specific concern, patients were evaluated by physical/occupational therapy as well as clinical nutrition. As a part of their nutritional evaluation, patients received education about cancer-related nutrition as well as various dietary supplements known to help with AEs of cancer treatment and to reduce future cancer risk. In addition, as part of the IO clinic and the YWBP, all patients were provided access to free fitness classes, gym referrals, and information regarding cancer-related physical activity.25,26
Chemotherapy-induced nausea and vomiting (CINV) during treatment for breast cancer is common and was reported as a reason for IO consultation by 20% of patients in our study. These symptoms are typically related to chemotherapy and can present in both an acute and delayed fashion. Acupuncture has demonstrated promising results for the treatment of CINV, so patients presenting with CINV were all referred for acupuncture as an adjunct to antiemetics.27-30 Chemotherapy-induced peripheral neuropathy (CIPN) is also a common AE of chemotherapy, especially with taxane use. A total of 17% of patients presented to the IO clinic with issues related to CIPN and were referred for acupuncture, as studies have shown promising outcomes with this modality.31,32 Further, many patients who are undergoing or have completed treatment experience treatment-related pain, including both acute surgical pain as well as chronic nonsurgical pain related to other aspects of their treatment (radiation, chemotherapy, aromatase inhibitors, etc). Although less common in our patient population, 5% of patients seen in our IO clinic endorsed pain as a concern and were offered healing touch or acupuncture, as these modalities have been shown to effectively treat acute, chronic, surgical, and nonsurgical cancer-related pain.33-35
While the diagnosis of any cancer is emotionally distressing for the patient, studies have shown that the experience for women diagnosed with breast cancer is particularly emotionally devastating and can lead to significant anxiety, depression, insomnia, and other mood disturbances.36 This was true for patients in our study as well, with 22% of patients endorsing insomnia, 9% anxiety, and 5% mood disturbances. These patients were provided a multitude of services such as meditation, yoga classes, guided relaxation, massage therapy, and music therapy, which have all been shown to have both short- and long-term benefits for those actively undergoing treatment of their breast cancer as well as those who are in remission.37-55
Timing of IO Treatment in Relation to Standard Cancer Care
Currently, there is a paucity of literature regarding the optimal timing of IO treatment with regard to standard cancer care. While patients were referred for IO consultation prior to initiating treatment, 69% did not participate in an IO consultation until after initiating their treatment. This is likely due to the symptom-specific treatment modalities offered by IO, and the symptoms may not emerge until treatment has begun. Currently, nothing in the literature supports empirically utilizing various IO treatment modalities prophylactically prior to patients exhibiting symptoms; however, the information from this study can be utilized in future studies to determine the optimal IO referral timing as it pertains to treatment and potentially the prevention of treatment-related symptoms. As such, this study serves as the groundwork at our institution for creation of guidelines regarding timing of IO therapies. Much like work being done in the prehabilitation world of general surgery, this area has the potential to significantly impact patient care by identifying ways to refer patients for IO consultation before treatment for breast cancer with the aim of prevention rather than treatment. Our goal in caring for this population of women is to ensure that IO services are available and offered to all patients.56,57
Limitations
While this study provides data regarding our current referral pattern to IO and the utilization of IO treatment modalities, there are also several limitations. While we know from our data that 12% of patients 40 years or younger utilized IO services, due to restrictions in the electronic medical record (EMR) system utilized by our cancer center during the time of this study, we were unable to track how many young women with new breast cancer diagnoses were referred to IO. For this reason, we could not determine the IO referral rate or how many patients who were referred to IO subsequently participated in a consultation. Given the importance of this information and the implications of referral rate vs actual appointment completion on patient care, we are working with our new EMR system to have access to this information for future studies. While 12% seems like a low rate of IO utilization, little in the literature exists regarding what rate would be appropriate or acceptable. Furthermore, the IO department at our institution was established relatively recently, in 2013, and it is likely that referral patterns have increased over time. Further defining this rate with the information from our study will help not only our institution, but the field of breast oncology generally to establish a baseline referral rate. In turn, this may facilitate strategic planning to improve referral rates and identify barriers experienced by referred patients who never utilized the IO services. Due to our data limitations, we were unable to discern the various treatment modalities utilized based on referral reason or presenting symptom. Additionally, symptom evolution was not tracked over time, so we were unable to assess if IO interventions had a positive impact on the initial symptom(s) prompting IO consultation. However, identification of these limitations has allowed us to correct some of these factors to be improved for future patient encounters. Future studies could assess referral patterns and track symptom improvement or worsening after incorporation of IO treatments into patients’ standard cancer care.
Conclusions
Our study aimed to retrospectively examine the use of IO therapies in a population of women 40 years and younger undergoing breast cancer treatment in our cancer center and to examine the timing of therapy use. Our data show that young women sought out a consultation by an IO physician to address TRAEs and a desire to decrease their future cancer risk. The most utilized therapies were acupuncture, massage, and healing touch, and more than 75% of those who sought treatment did so after starting SOC therapy. Although the extent to which integrative therapies may affect a clinically relevant change in TRAEs or future cancer risk remains unknown, this study serves to present preliminary data to support more rigorous future studies examining the optimal timing of integrative therapies as well as the specific integrative modalities that may be associated with improved outcomes. Recent improvements to our EMR system will facilitate referral pattern tracking in the future. Our data may be used to guide conversations when referring young women to IO and, as a result, better define patterns of IO referral and optimize the various IO treatment resources. This will enable us to deliver the most holistic care possible for our patients. Furthermore, these data could serve as a precursor to development of algorithms/pathways within integrative oncology to prevent AEs rather than treat them. This could greatly impact the overall quality of life of patients in addition to leading to a cost savings to our health care systems.
These data were previously presented as an abstract at the 2021 American Society of Clinical Oncology Annual Meeting.
FINANCIAL SUPPORT: Donated by the Levine Family and the Sandra Levine Young Women’s Breast Cancer Program.
ACKNOWLEDGMENT: We thank Michelle L. Wallander, PhD, who provided medical writing support on behalf of the Department of Surgery, Carolinas Medical Center, Charlotte, NC.
FINANCIAL DISCLOSURES: JF reports receiving honoraria from Healio.
AUTHOR AFFILIATIONS:
Yancey Warren, Jr, MD, MAT1; Anna Hecksher, BS2; Courtney Schepel, BS2; Sally Trufan, MS3; Rebecca Greiner, PA4; Susan Yaguda, MSN, RN4; Chasse Bailey-Dorton, MD, MSPH4; Julie Fisher, MD5; Richard White, MD1; and Lejla Hadzikadic-Gusic, MD, MSc1
1Department of Surgery, Levine Cancer Institute, Carolinas Medical Center, Atrium Health, Charlotte, NC
2Sandra Levine Young Women’s Breast Cancer Program, Levine Cancer Institute, Charlotte, NC
3Department of Cancer Biostatistics, Levine Cancer Institute, Charlotte, NC
4Department of Supportive Oncology, Levine Cancer Institute, Charlotte, NC
5Department of Medical Oncology, Levine Cancer Institute, Charlotte, NC
CORRESPONDING AUTHOR
Lejla Hadzikadic-Gusic, MD, MSc
Division of Surgical Oncology
Levine Cancer Institute & Department of Surgery
Carolinas Medical Center
1021 Morehead Medical Dr
Suite 6200
Charlotte, NC
Phone: 980-442-6357
Fax: 980-442-6321
Email: Lejla.Hadzikadic-Gusic@atriumhealth.org
References
- Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36(3):237-249. doi:10.1053/j.seminoncol.2009.03.001
- DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31-42. doi:10.3322/caac.21320
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-2917. doi:10.1002/ijc.25516
- Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4(11):e7695. doi:10.1371/journal.pone.0007695
- Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208(3):341-347. doi:10.1016/j.jamcollsurg.2008.12.001
- Keegan THM, DeRouen MC, Press DJ, Kurian AW, Clarke CA. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14(2):R55. doi:10.1186/bcr3156
- Swain SM, Nunes R, Yoshizawa C, Rothney M, Sing AP. Quantitative gene expression by recurrence score in ER-positive breast cancer, by age. Adv Ther. 2015;32(12):1222-1236. doi:10.1007/s12325-015-0268-3
- Davies MC, Hall ML, Jacobs HS. Bone mineral loss in young women with amenorrhoea. Bmj. 1990;301(6755):790-793. doi:10.1136/bmj.301.6755.790
- Elkum N, Dermime S, Ajarim D, et al. Being 40 or younger is an independent risk factor for relapse in operable breast cancer patients: the Saudi Arabia experience. BMC Cancer. 2007;7:222. doi:10.1186/1471-2407-7-222
- Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21(22):4184-4193. doi:10.1200/jco.2003.04.196
- Han W, Kang SY; Korean Breast Cancer Society. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat. 2010;119(1):193-200. doi:10.1007/s10549-009-0388-z
- Petkov VI, Miller DP, Howlader N, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer. 2016;2:16017. doi:10.1038/npjbcancer.2016.17
- Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120-4128. doi:10.1200/jco.2009.25.9655
- Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602-606. doi:10.1200/jco.2003.07.071
- Poorvu PD, Partridge AH. Epidemiology. In: Gentilini O, Partridge AH, Pagani O, eds. Breast cancer in young women. Springer; 2020:1-12.
- Greenlee H, Balneaves LG, Carlson LE, et al. Society for Integrative Oncology. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. J Natl Cancer Inst Monogr. 2014;2014(50):346-358. doi:10.1093/jncimonographs/lgu041
- Latte-Naor S, Mao JJ. Putting integrative oncology into practice: Concepts and approaches. J Oncol Pract. 2019;15(1):7-14. doi:10.1200/jop.18.00554
- Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Society for Integrative Oncology. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67(3):194-232. doi:10.3322/caac.21397
- Wiśniewska I, Jochymek B, Lenart-Lipińska M, Chabowski M. The pharmacological and hormonal therapy of hot flushes in breast cancer survivors. Breast Cancer. 2016;23(2):178-182. doi:10.1007/s12282-015-0655-2
- Morrow PKH, Mattair DN, Hortobagyi GN. Hot flashes: a review of pathophysiology and treatment modalities. Oncologist. 2011;16(11):1658-1664. doi:10.1634/theoncologist.2011-0174
- Bokmand S, Flyger H. Acupuncture relieves menopausal discomfort in breast cancer patients: a prospective, double blinded, randomized study. Breast. 2013;22(3):320-323. doi:10.1016/j.breast.2012.07.015
- Deng G, Vickers A, Yeung S, et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J Clin Oncol. 2007;25(35):5584-5590. doi:10.1200/jco.2007.12.0774
- Hervik J, Mjåland O. Quality of life of breast cancer patients medicated with anti-estrogens, 2 years after acupuncture treatment: a qualitative study. Int J Womens Health. 2010;2:319-325. doi:10.2147/ijwh.S12809
- Liljegren A, Gunnarsson P, Landgren BM, Robéus N, Johansson H, Rotstein S. Reducing vasomotor symptoms with acupuncture in breast cancer patients treated with adjuvant tamoxifen: a randomized controlled trial. Breast Cancer Res Treat. 2012;135(3):791-798. doi:10.1007/s10549-010-1283-3
- Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical activity, and Cancer: impact and future directions. J Nutr. 2020;150(4):663-671. doi:10.1093/jn/nxz268
- Thomas RJ, Kenfield SA, Jimenez A. Exercise-induced biochemical changes and their potential influence on cancer: a scientific review. Br J Sports Med. 2017;51(8):640-644. doi:10.1136/bjsports-2016-096343
- Dibble SL, Luce J, Cooper BA, et al. Acupressure for chemotherapy-induced nausea and vomiting: a randomized clinical trial. Oncol Nurs Forum. 2007;34(4):813-820. doi:10.1188/07.ONF.xxx-xxx
- Ezzo J, Vickers A, Richardson MA, et al. Acupuncture-point stimulation for chemotherapy-induced nausea and vomiting. J Clin Oncol. 2005;23(28):7188-7198. doi:10.1200/jco.2005.06.028
- Garcia MK, McQuade J, Haddad R, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013;31(7):952-960. doi:10.1200/jco.2012.43.5818
- Molassiotis A, Helin AM, Dabbour R, Hummerston S. The effects of P6 acupressure in the prophylaxis of chemotherapy-related nausea and vomiting in breast cancer patients. Complement Ther Med. 2007;15(1):3-12. doi:10.1016/j.ctim.2006.07.005
- Lu W, Dean-Clower E, Doherty-Gilman A, Rosenthal DS. The value of acupuncture in cancer care. Hematol Oncol Clin North Am. 2008;22(4):631-648, viii. doi:10.1016/j.hoc.2008.04.005
- Paley CA, Johnson MI, Tashani OA, Bagnall AM. Acupuncture for cancer pain in adults. Cochrane Database Syst Rev. 2015;(10):CD007753. doi:10.1002/14651858.CD007753.pub3
- Chen L, Lin CC, Huang TW, et al. Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: a meta-analysis of randomized controlled trials. Breast. 2017;33:132-138. doi:10.1016/j.breast.2017.03.015
- Crew KD, Capodice JL, Greenlee H, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol. 2010;28(7):1154-1160. doi:10.1200/jco.2009.23.4708
- Mao JJ, Xie SX, Farrar JT, et al. A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer. 2014;50(2):267-276. doi:10.1016/j.ejca.2013.09.022
- Campbell-Enns H, Woodgate R. The psychosocial experiences of women with breast cancer across the lifespan: a systematic review protocol. JBI Database System Rev Implement Rep. 2015;13(1):112-121. doi:10.11124/jbisrir-2015-1795
- Lyman GH, Bohlke K, Cohen L. Integrative therapies during and after breast cancer treatment: Asco endorsement of the SIO Clinical Practice Guideline Summary. Journal of Oncology Practice. 2018;14(8):495-499. doi:10.1200/jop.18.00283
- Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766-3775. doi:10.1002/cncr.26702
- Rao MR, Raghuram N, Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complement Ther Med. 2009;17(1):1-8. doi:10.1016/j.ctim.2008.05.005
- Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009;8(1):37-46. doi:10.1177/1534735409331456
- Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6(3):242-250. doi:10.1177/1534735407306214
- Binns-Turner PG, Wilson LL, Pryor ER, Boyd GL, Prickett CA. Perioperative music and its effects on anxiety, hemodynamics, and pain in women undergoing mastectomy. AANA J. 2011;79(4 Suppl):S21-S27.
- Dhruva A, Miaskowski C, Abrams D, et al. Yoga breathing for cancer chemotherapy-associated symptoms and quality of life: results of a pilot randomized controlled trial. J Altern Complement Med. 2012;18(5):473-479. doi:10.1089/acm.2011.0555
- Hanser SB, Bauer-Wu S, Kubicek L, et al. Effects of a music therapy intervention on quality of life and distress in women with metastatic breast cancer. J Soc Integr Oncol. 2006;4(3):116-124. doi:10.2310/7200.2006.014
- Kim YH, Kim HJ, Ahn SD, Seo YJ, Kim SH. Effects of meditation on anxiety, depression, fatigue, and quality of life of women undergoing radiation therapy for breast cancer. Complement Ther Med. 2013;21(4):379-387. doi:10.1016/j.ctim.2013.06.005
- Fernández-Lao C, Cantarero-Villanueva I, Díaz-Rodríguez L, Cuesta-Vargas AI, Fernández-Delas-Peñas C, Arroyo-Morales M. Attitudes towards massage modify effects of manual therapy in breast cancer survivors: a randomised clinical trial with crossover design. Eur J Cancer Care (Engl). 2012;21(2):233-241. doi:10.1111/j.1365-2354.2011.01306.x
- Li X-M, Zhou K-N, Yan H, Wang D-L, Zhang Y-P. Effects of music therapy on anxiety of patients with breast cancer after radical mastectomy: a randomized clinical trial. J Adv Nurs. 2012;68(5):1145-1155. doi:10.1111/j.1365-2648.2011.05824.x
- Billhult A, Bergbom I, Stener-Victorin E. Massage relieves nausea in women with breast cancer who are undergoing chemotherapy. J Altern Complement Med. 2007;13(1):53-57. doi:10.1089/acm.2006.6049
- Hernandez-Reif M, Ironson G, Field T, et al. Breast cancer patients have improved immune and neuroendocrine functions following massage therapy. J Psychosom Res. 2004;57(1):45-52. doi:10.1016/s0022-3999(03)00500-2
- Kovačič T, Zagoričnik M, Kovačič M. Impact of relaxation training according to the Yoga In Daily Life® system on anxiety after breast cancer surgery. J Complement Integr Med. 2013;10:/j/jcim.2013.10.issue-1/jcim-2012-0009/jcim-2012-0009.xml. doi:10.1515/jcim-2012-0009
- Listing M, Krohn M, Liezmann C, et al. The efficacy of classical massage on stress perception and cortisol following primary treatment of breast cancer. Arch Womens Ment Health. 2010;13(2):165-173. doi:10.1007/s00737-009-0143-9
- Molassiotis A, Yung HP, Yam BMC, Chan FYS, Mok TSK. The effectiveness of progressive muscle relaxation training in managing chemotherapy-induced nausea and vomiting in Chinese breast cancer patients: a randomised controlled trial. Support Care Cancer. 2002;10(3):237-246. doi:10.1007/s00520-001-0329-9
- Listing M, Reisshauer A, Krohn M, et al. Massage therapy reduces physical discomfort and improves mood disturbances in women with breast cancer. Psychooncology. 2009;18(12):1290-1299. doi:10.1002/pon.1508
- Post-White J, Kinney ME, Savik K, Bernsteen Gau J, Wilcox C, Lerner I. Therapeutic massage and healing touch improve symptoms in cancer. Integr Cancer Ther. 2003;2(4):332-344. doi:10.1177/1534735403259064
- Wilkinson SM, Love SB, Westcombe AM, et al. Effectiveness of aromatherapy massage in the management of anxiety and depression in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(5):532-539. doi:10.1200/jco.2006.08.9987
- Wischmeyer PE, Carli F, Evans DC, et al; Perioperative Quality Initiative (POQI) 2 Workgroup. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Nutrition Screening and Therapy Within a Surgical Enhanced Recovery Pathway. Anesth Analg. 2018;126(6):1883-1895. doi:10.1213/ane.0000000000002743. Published correction appears in Anesth Analg. 2018;127(5):e95
- Wynter-Blyth V, Moorthy K. Prehabilitation: preparing patients for surgery. BMJ. 2017;358:j3702. doi:10.1136/bmj.j3702
