Lower Dose Capecitabine Is Active and Has Favorable Safety Profile in Elderly Patients With Advanced Breast Cancer
This special supplement to Oncology News International includes 28 reportswith updated information on clinical trials investigating capecitabine and other agents inthe treatment of advanced colorectal and breast cancers, and other solid tumors.The reports summarize selected presentations from the 39th Annual Meeting of theAmerican Society of Clinical Oncology (ASCO) and related educational symposiaheld in conjunction with ASCO.
MILAN, Italy-A reduced doseof oral capecitabine (Xeloda) is a welltolerated,effective, and convenient treatmentfor elderly patients with advancedbreast cancer.These results of an Italian study werereported by one of the investigators, LauraCatena, MD, Istituto Nazionale per loStudio e la Cura dei Tumori, Milan, Italy(ASCO abstract 3050)."There is a need for a well-toleratedand effective treatment in elderly breastcancer patients. Half of all breast cancersare in elderly women, and many treatmentsfor advanced breast cancer arepoorly tolerated by elderly patients," Dr.Catena explained."Oral capecitabine enables convenient,outpatient treatment. It achieves consistentlyhigh efficacy in metastatic breastcancer, with a favorable safety profile characterizedby a particularly low incidenceof grade 3/4 adverse events, myelosuppression,and alopecia." Capecitabine, atumor-activated, oral fluoropyrimidine,generates fluorouracil (5-FU) preferentiallyin tumor tissue, and presents goodactivity in a wide range of solid tumors.25% Dose ModificationThe open-label, noncomparative,phase II study was conducted at a singlecenter between May 1999 and February2003. The study evaluated the global therapeuticindex of twice-daily oral capecitabinein elderly patients with locally advancedmetastatic breast cancer.Patients received oral capecitabine1,250 mg/m2 bid, days 1 to 14 every 21days (n = 30). To improve the safety profile,the protocol was amended to a reduceddosage of oral capecitabine 1,000mg/m2 bid, days 1 to 14 every 21 days(n = 43). This reduction was in responseto new dosing guidelines recommendinga 25% dose reduction in elderly, frail patientswho may be at risk of an increasedincidence of grade 3/4 toxicities.
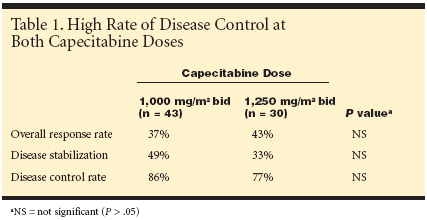
Patients with a partial response or stabledisease had up to six cycles of treatment;patients with complete responsehad an additional two cycles, and patientswith progressive disease were withdrawnfrom the study.A total of 73 patients were enrolled,and the median age of the patients was72.9 years (range 65 to 89). All patientshad measurable or evaluable advanceddisease, Karnofsky performance status≤ 2, and adequate bone marrow, cardiac,renal, and hepatic function.Patients could have had no more thanone prior chemotherapy regimen and/ortwo hormonal regimens for metastaticdisease. A previous therapy containingfluorouracil was allowed but a minimumperiod of 12 months was required startingfrom the last dosage of the previous treatment.The metastatic sites were soft tissue(28), bone (27), liver (26), lung (20), andothers (20). The primary end points werethe safety profile and tolerability, the secondaryend points were response rate andtime to progression.Most Completed Full CourseOverall, 39 patients (53%) completedthe full course of treatment. There was asimilar incidence of premature withdrawalat the two treatment doses, and treatment-related adverse events led to prematurewithdrawal of 13 patients (18%).All patients were evaluable for toxicityand 62 patients for response. Toxicityaccording to National Cancer Institute-Common Toxicity Criteria (NCI-CTC)Bethesda guidelines was: grade 3/4 diarrhea(6%), grade 3 vomiting (7%), grade1/2 hand-foot syndrome (47% in thehigher-dose group and 30% in the lowdosegroup), grade 2/3 asthenia (26%),grade 2 stomatitis (11%). There were twodeaths during the study; one due to treatmentrelated diarrhea/dehydration, andone due to disease progression."There were fewer dose reductions foradverse events required in patients receivingthe lower dose compared with patientsreceiving the higher dose," noted thestudy's lead investigator, Giuseppe Procopio,MD, of Istituto Nazionale per lo Studioe la Cura dei Tumori, Milan, Italy.Considerable Activity"Oral capecitabine demonstrated considerableactivity, achieving a high rate ofdisease control, with no significant differencesbetween the lower and higher dosegroups," Dr. Procopio continued. Diseasecontrol was 86% with the lower dose and77% with the higher dose (see Table 1).Objective responses were documented in36% of patients (26), with 3% completeremission, 32% with stabilization ofdisease, and 18% with progression. Themedian time to disease progression was77 days (range 14-139 days).Dr. Catena reported that the studyfound a favorable safety profile of oralcapecitabine 1,000 mg/m2 twice daily inelderly patients compared to the higherdose. "There was a low incidence of grade3/4 adverse events and no grade 3/4myelosuppression. There was a lower incidenceof grade 3/4 diarrhea (2% vs 13%)and fewer dose reductions (2% vs 30%)compared with oral capecitabine 1,250mg/m2 twice daily."This lower dose regimen is as effectiveand better tolerated than the higher doseregimen and can be recommendedfor elderly patients with locallyadvanced metastatic breast cancer," Dr.Catena concluded.