Lymphedema: Separating Fact From Fiction
Given the abundance of breast cancer data, this review will focus on breast cancer–related lymphedema. However, the principles and controversies discussed are relevant regardless of the type of malignancy to which the lymphedema is attributed.
Lymphedema is a feared complication of cancer treatment and one that negatively impacts survivorship. The incidence of breast cancer–related lymphedema ranges from 6% to 70%, but lymphedema may be a common and under-reported morbidity. No standard guidelines for its diagnosis and assessment exist. Although the true etiology of lymphedema remains unknown, radiation, chemotherapy, type of breast surgery, and extent of axillary surgery are commonly cited risk factors. However, the relationship between the number of nodes removed and the risk of lymphedema is not clearly correlated. Clinical trials are focusing on ways to reduce the need for axillary dissection even in the setting of a positive sentinel node, to help minimize axillary morbidity. Risk-reduction practices, including avoidance of skin puncture and blood pressures in the ipsilateral upper extremity, and precautionary behaviors such as wearing compression garments during air travel continue to be advocated by the medical and survivor communities, despite a lack of rigorous evidence supporting their benefit. Emerging data support exercise in at-risk and affected women with lymphedema when started gradually and increased cautiously.
Introduction
The most recent survivorship data from the Surveillance, Epidemiology, and End Results (SEER) database demonstrate a significant increase in the number of US cancer survivors over the last 30 years, from roughly 3 million in 1973 to nearly 12 million in 2008.[1] As a result, issues of survivorship have stimulated new focus for clinical trials, not only to determine the most effective therapeutic regimen (surgery, drug, or radiation) but also to identify the one with the least influence on future quality of life. Lymphedema has long been a feared complication of surgical cancer treatment, and notably one that negatively impacts survivorship. Fear of lymphedema stems from patient concerns regarding the chronic, progressive nature of the condition and the clinician’s relative inability to predict or prevent its development. Furthermore, decades of physician and allied health teachings based on opinion and theory have perpetuated the myths shrouding lymphedema risk, prevention, and treatment.
A vast body of literature documents the occurrence of breast cancer–related lymphedema, with more than 1400 articles indexed in PubMed-MEDLINE databases alone. Importantly, lymphedema also exists after surgery for non–breast-cancer-related malignancies, but data documenting this occurrence are rare in comparison. Recently, Cormier et al found only 47 studies between 1972 and 2008 with more than 10 patients that prospectively evaluated lymphedema as a primary or secondary outcome after treatment for melanoma, bladder, sarcoma, penile, prostate, vulvar, cervical, endometrial, or head and neck cancers.[2] The authors’ analysis of these studies demonstrated the overall incidence of lymphedema to be 16.3% after melanoma, 10.1% after genitourinary cancers, and 19.6% after gynecologic malignancies, and notes that lymphedema rates are higher when the lower rather than upper extremity is affected. Given the abundance of breast cancer data, this review will focus on breast cancer–related lymphedema. However, the principles and controversies discussed are relevant regardless of the type of malignancy to which the lymphedema is attributed.
Incidence and Diagnosis
The incidence of breast cancer–related lymphedema has been difficult to quantify due to delayed onset of symptoms and the lack of standardized diagnostic criteria. A recent meta-analysis reports the incidence of breast cancer–related lymphedema to range from 0 to 3% after lumpectomy alone to as high as 65% to 70% after modified radical mastectomy (removal of breast and axillary lymph nodes) with regional nodal radiation.[3] Overall, 80% to 90% of women who will develop lymphedema do so within 3 years of treatment,[4,5] but the risk persists years later as the remaining 10% to 20% will develop lymphedema at a rate of 1% per year.[5] Petrek et al followed 263 patients and found that nearly 50% developed lymphedema by 20 years.[5] These data suggest that lymphedema is probably more common than generally reported, and clearly the length of follow-up in a given study influences the reported incidence. A uniform definition of lymphedema does not exist. Although volume displacement methods are considered the gold standard for diagnosis, these methods are cumbersome and unable to identify subclinical lymphedema. Thus, displacement methods are rarely used in clinical settings. Circumferential arm measurements performed with a nonelastic tape measure are commonly used to determine upper extremity size differences. It is essential to obtain baseline preoperative measurements of the ipsilateral and contralateral extremities, as differences of up to 2 cm may naturally exist between the dominant and nondominant arms at baseline.[6] Published literature demonstrates significant heterogeneity, as studies lack agreement on the location and minimal number of measurements performed. Furthermore, they use multiple definitions of what constitutes lymphedema, including measurement changes greater than 2 cm, volume increase of more than 200 mL, or percentage increases in volume (> 10% or > 20%) compared with baseline when controlled for changes in the contralateral arm.[7,8] Aside from objective measurements, some studies define lymphedema by patient subjective symptoms or patient perceptions of swelling.
Controversy remains as to whether a measurement change or a patient’s perception of swelling should be considered diagnostic. While a measurement change is objective, measurements may be affected by inter-rater and even intra-rater variability affecting reproducibility. Perhaps more importantly, a defined measurement change may be asymptomatic in an obese patient, while a thin woman may be bothered by more subtle measurement changes not meeting measured criteria for lymphedema. Alternatively, a patient’s perception of swelling may more accurately diagnose symptomatic lymphedema, but this perception may be influenced by sensory changes from the surgery and additional adjuvant therapies. Further, women with surgery in their dominant arm may perceive functional issues to a greater degree than if it were the nondominant arm that was affected.[9] In fact, one study found only 41% of women with perceived swelling had circumferential arm measurement changes greater than 2 cm, the objective threshold used in the study to define lymphedema.[9] Several contemporary studies have evaluated the differences between measured and perceived lymphedema and have validated patient perceptions against physical therapist–directed measurements.[4,10] These studies conclude that each evaluation method is valid but not interchangeable, and they do not endorse one method over another.
The clinical introduction of a single-frequency bioelectrical impedance device manufactured by Impedimed (Mansfield, Australia) can minimize both inter-rater and intra-rater variability seen with other measurement techniques. The device produces easily interpretable values measuring the changes in extracellular fluid when compared with the unaffected contralateral upper extremity. A score increase of 10 or more or one that registers outside the normal range should prompt intervention with a compression garment or a referral for physical therapy. Some investigators report that measurement of bioimpedance has improved sensitivity in detecting subclinical lymphedema up to 4 months prior to standard measurement changes.[11] For these reasons, the current National Lymphedema Network (NLN) breast cancer–related lymphedema guidelines recommend that all breast cancer patients receive pre- and post-treatment measurements (of any type) on both arms and encourage the use of bioimpedance spectroscopy or infrared perometry as alternatives to tape measures, to limit measurement variations.[12]
Is It All About the Nodes?
Over the years, many retrospective studies have reported risk factors for lymphedema. Axillary node dissection (ALND), mastectomy, obesity, radiation, infection, and ipsilateral upper extremity injury consistently rank as the most influential causes of damage or disruption to the lymphatic system and thus lymphedema. However, the magnitude of association between breast cancer treatment factors and lymphedema is inconsistent across studies.[8] Tsai et al reviewed 98 studies conducted in the United States and Canada through January 2008 for lymphedema risk factors.[8] The authors report a significantly increased risk for lymphedema in women undergoing mastectomy compared with lumpectomy (risk ratio [RR], 1.42; 95% confidence interval [CI], 1.15–1.76), ALND compared with no dissection (RR, 3.47; CI, 2.34–5.15), ALND compared with sentinel lymph node biopsy (SLNB) (RR, 3.07; CI, 2.20–4.29), radiation therapy vs no radiation therapy (RR, 1.92; CI, 1.61–2.28), and positive vs negative axillary lymph nodes (RR, 1.54; CI, 1.32–1.80). While this meta-analysis demonstrates a comprehensive, contemporary review of lymphedema risk factors, the heterogeneity of the data must be considered, as 11 different definitions for lymphedema were used and follow-up ranged between 1 month and 30 years.
Though the most intuitively obvious risk factor for lymphedema is the number of nodes removed, the relationship between the number of lymph nodes removed and the risk of lymphedema remains unresolved. Several retrospective studies have shown that the number of nodes removed and the risk of lymphedema do not correlate.[13,14] Others find an increasing risk of lymphedema as more nodes are removed.[15-17] Despite various lengths of follow-up, from 6 to 60 months, the prospective trials vetting SLNB as the standard of care for axillary staging demonstrate significantly reduced rates of lymphedema after SLNB (0 to 7%) compared with ALND (12% to 16%).[18-21] These studies certainly support the concept that the risk of lymphedema is proportional to the extent of axillary surgery. Importantly, though, they also confirm that even with SLNB a small but clear risk of lymphedema remains. A recent study by Goldberg et al suggests that it is not the number of lymph nodes removed but instead the degree of dissection and disruption of the lymphatic system that results in lymphedema.[22] The authors reviewed 600 women having SLNB with a median follow-up of 5 years and found an overall incidence of lymphedema of 5%. When stratifying the data according to the number of nodes removed, they found no significant association between the mean, median, or range of number of nodes excised and lymphedema (P = .93). Furthermore, the authors completed a subset analysis of the women having more than 10 lymph nodes removed at SLNB. None of these women developed lymphedema. Interestingly, when these SLNB patients having > 10 nodes removed were compared with a separate group of women having 10 to 17 nodes removed at ALND, 11% of the ALND patients had measured lymphedema (P = .04). The fact that women having more than 10 nodes removed during SLNB did not develop lymphedema but women with the same number of nodes removed after ALND did reaffirms that the relationship between the nodes removed and lymphedema is complex. Perhaps it is the relative magnitude of lymphatic destruction and individual patient ability to form collateral lymphatic channels, rather than the number of nodes removed, that influences lymphedema risk. For example, a patient with many nodes removed at SLNB and no finding of lymphedema may have more lymphatic collaterals and therefore will have suffered relatively less lymphatic disruption despite a larger than “normal” number of SLNs removed. On the other hand, women having ALND and a relatively small number of total nodes excised may have suffered an overall greater degree of lymphatic disruption and therefore develop lymphedema. Unfortunately, the number of nodes within each patient’s nodal basin and the patient’s ability to protect or form new lymphatic collaterals during or after treatments is unknown. Therefore, simply the number of nodes removed may be insufficient to determine lymphedema risk.
If, however, the degree of lymphatic disruption or damage is the key driver of lymphedema risk, then it is plausible that radiation can also act primarily or synergistically to influence lymphedema risk. Axillary radiation alone is not without complications, as these patients can have a 2- to 4.5-fold increase in the risk of lymphedema. A recent meta-analysis by Shah and Vicini finds lymphedema in 9% to 65% of patients after lumpectomy alone (no nodal surgery) and regional nodal radiation and in 58% to 65% of women after mastectomy alone and regional nodal radiation.[3] Additionally, the synergistic effect of surgery and radiation is well documented to result in a 3.5- to 10-fold higher risk of lymphedema when compared with surgery alone.[3,23,24]
Finally, accumulating data suggest that chemotherapy may also affect lymphatic destruction. Norman et al found anthracycline-based chemotherapy regimens increase the risk of lymphedema.[25] Their prospective review of 631 breast cancer survivors followed for 5 years found an elevated hazard ratio (HR) of 1.46 (95% CI, 1.04–2.04) for lymphedema among breast cancer patients receiving anthracycline-based chemotherapy vs no chemotherapy. This risk persisted after stratifying for stage at diagnosis or number of positive nodes. Furthermore, the authors concluded that treatment combinations involving ALND or chemotherapy resulted in approximately four- to five-fold increases in the HRs for lymphedema (HR, 4.16 [95% CI, 1.32–12.45]for SLNB/chemotherapy/no radiation)compared with no treatment. While further validation of this finding is needed, the concept that chemotherapy independently influences lymphedema risk demonstrates that this risk can be affected not only by locally directed therapies but also by systemic ones.[25]
Changes in Surgical Management
Changes in the surgical management of the axilla have been instrumental in reducing axillary morbidity, especially lymphedema. SLNB is now the standard of care for axillary staging, as its accuracy, low false-negative rate, and low rate of axillary recurrence have been documented in more than 70 studies. Current research to further minimize morbidity from axillary surgery focuses on reducing the need for completion axillary dissection in the setting of a positive axillary node. The American College of Surgeons (ACOSOG) Z0011 trial published in early 2011 documents the first prospective randomized data showing that at a median of 6 years follow-up, SLNB alone does not result in inferior survival in women with T1 or T2 tumors and one or two positive sentinel nodes who received breast conservation surgery, chemotherapy and/or hormonal therapy, and whole breast radiation therapy.[26] In the study, more than 70% of the women with a positive SLNB had no additional positive nodes, suggesting that in select cases SLNB can be both diagnostic and therapeutic. While this trial is viewed as practice-changing, it is important to note that the trial called for standard whole breast radiation but did not standardize the radiation tangents other than to say that third field–directed axillary radiation was forbidden. As a result it is possible that radiation tangents were adjusted higher in node-positive women who were randomized to the SLNB-only arm. If standard radiation tangents cover approximately 30% to 50% of the level I and 25% of the level II axillary nodes,[27] then adjusting the tangents “higher” has the intention of covering more nodes than standard. These data are important from the perspective of morbidity, as an adjustment in the tangents may result in increased rates of axillary morbidity above what is seen with standard whole breast tangents and SLNB alone. The radiation ports are currently being evaluated retrospectively. An additional prospective trial, ACOSOG Z1071, is evaluating the validity, accuracy, and false-negative rate of SLNB after neoadjuvant chemotherapy in women who present with node-positive disease at diagnosis. The investigators hypothesize that SLNB after neoadjuvant chemotherapy will appropriately and reliably stage the axilla and therefore allow elimination of ALND in women converted by neoadjuvant chemotherapy from node-positive to node-negative status. The trial accrued quickly and results are pending. Remaining questions to be answered include that of which women may be able to forgo axillary surgery (SLNB or ALND) altogether.
Risk-Reduction Practices
As long as axillary surgery and/or radiation remain pillars of breast cancer treatment, lymphedema will remain a potential complication. Risk-reduction practices to further prevent lymphedema after axillary surgery have long been advised. It is here that many of the myths regarding lymphedema risk and prevention arise. The National Lymphedema Network, founded in 1988, seeks to educate and guide patients and healthcare providers about lymphedema and risk-reduction practices for those at risk for and affected by the disorder. The organization publishes guidelines on risk-reducing behaviors and seeks to answer the question of whether behavioral modifications can further limit or reduce lymphedema. The NLN’s risk-reduction guidelines were updated in 2011 and continue to carry a disclaimer stating that “there is little evidence-based literature regarding many of these practices, [therefore] the majority of the recommendations must at this time be based on the knowledge of pathophysiology and decades of clinical experience by experts in the field.”[12] Although the National Comprehensive Cancer Network (NCCN) and the National Cancer Institute (NCI) do not publish specific guidelines regarding lymphedema diagnosis, treatment, or prevention, the NCCN and NCI websites for patients both describe and define lymphedema and endorse precautionary behaviors similar to those recommended by the NLN.[28,29] The primary goal of the risk-reducing measures is to prevent overload of the lymphatic system by limiting activities that may increase lymphatic flow, local blood flow, or metabolic waste products in the at-risk or affected limb. The recommended behaviors support activity, medical procedure, and lifestyle modifications. Although the lymphedema risk-reduction strategies promulgated by these national organizations are based on sound physiologic principles, the effect of each recommended measure on preventing lymphedema is unclear, as is the impact on one’s quality of life if these measures are followed. Although Boccardo et al documented that increased education and awareness can reduce the incidence of lymphedema,[30] it is also possible that increased education can influence patient anxiety. One study reviewed factors affecting patients’ intention to avoid strenuous activity by following 175 women for a median of 10 months after axillary surgery. The authors found women given advice on arm care and those with perceived vulnerability regarding, and fear of, lymphedema were more likely to avoid using their arm (odds ratio [OR], 4.9; 95% CI, 1.73–14.06; P = .003; and OR 1.6, 95% CI 1.18–2.16, P = .003, respectively). Interestingly, however, the extent of axillary surgery did not influence the women’s arm use (OR, 1.3; 95% CI, 0.56–3.11; P = .52).[31]
Several additional inconsistencies exist regarding risk-reduction behaviors. First, standardized advice does not meet the needs of all patients, especially given that the recommendations do not always differentiate between at-risk and affected individuals. Second, there is inconsistent application of the practices across disease sites. While these recommendations are frequently discussed in relation to breast cancer treatment, they are rarely discussed within the context of other malignancies that require lymph node assessment or treatment. Further, many additional patients annually undergo lymph node excision for diagnosis of lymphoma or infection. Ironically, they leave the hospital without having discussed future lymphedema risk with their physicians, despite having a number of lymph nodes removed that is comparable to that of a patient undergoing SLNB for breast cancer. Third, there is still debate over what constitutes a risk-reducing practice. Patients recognize these controversies, given that they frequently receive inadequate or conflicting advice regarding arm care.[31]
TABLE 1
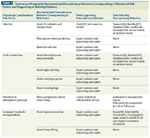
Summary of Frequently Recommended Precautionary Behaviors Corresponding to Theoretical Risk Factors and Supporting or Refuting Evidence
Despite these inconsistencies, breast cancer survivors have adopted many of the existing lymphedema risk–reducing practices. A prospective study of nearly 1000 breast cancer survivors found that patients having ALND practice a mean of 5.1 risk-reducing behaviors, while those having SLNB follow 4.3.[9] Although this difference was statistically significant (P < .0001), one could argue that it is not clinically significant. For example, more than 98% of women who have undergone ALND avoid blood pressures, intravenous catheter placement, and needle sticks in the ipsilateral arm, while more than 80% of SLNB patients do the same without documented benefit and theoretically less risk than those having ALND. Examination of the recommended risk-reducing behaviors reveals few objective data for or against each measure. The data regarding commonly considered precautionary behaviors are summarized in Table 1. An in-depth review of the role of compression garments when flying, and of IV placement, needle sticks, blood pressures, and exercise, follows.
The physiologic theory supporting the role of compression garments during air travel stems from the low cabin pressure that exists during flight, which causes a decrease in extracellular fluid pressure. This pressure decrease facilitates escape of fluid and proteins from the lymphatic vasculature, resulting in lymphedema. A literature review finds only a single retrospective survey–based study of patients with lymphedema published more than 15 years ago, in which 27 of 490 (5.5%) patients surveyed link the onset or worsening of their lymphedema to an airplane flight. The authors of this study cited lowered cabin pressure as the cause but do note the lack of direct evidence.[32] Based on this report and its suggested physiologic premise, at-risk and affected patients are recommended to wear a compression garment, to help regulate the extracellular pressure and support the lymphatic musculature maintaining lymphatic flow.[12] Limited contemporary data, however, do not appear to support these findings or the use of compression garments. Graham et al retrospectively surveyed 287 at-risk survivors regarding air travel.[33] Overall, 50% of them traveled a mean of five flights, and of this group 86% practiced precautionary behaviors consisting of routine use of compression garments with or without other behaviors. The authors observed no difference in lymphedema rates between fliers and nonfliers (P = .42), but interestingly they found the practice of precautionary behaviors to be associated with an increased risk of lymphedema (OR, 6.2; 95% CI, 1.2–20.8; P < .04) among those flying. Furthermore, when analyzed independently, the use of compression garments appeared not to correlate with other suspected lymphedema risk factors, including nodal disease, number of nodes removed, or radiation. They concluded that garments were not necessary and might be counterproductive.[32] Kilbreath et al drew similar conclusions after they prospectively evaluated 72 women preflight and 6 weeks after planned international or transcontinental air travel. Bioimpedance analysis in one study found no difference in extracellular fluid content between baseline and follow-up, regardless of flight distance or compression garment use. Despite their short follow-up and the fact that the women followed were athletes (which may have a protective effect), the authors concluded that air travel did not cause lymphedema.[34]
Another long-supported myth regarding prevention of lymphedema is the avoidance of IV catheters or needle sticks. The theory behind this practice arises from the possible risk of infection produced by accidental and nonaccidental skin punctures and is less concerned with the actual puncture itself. Infection will cause an intense inflammatory response, altering the extremity fluid homeostasis. Despite the longevity of this landmark recommendation, a review of the literature finds only one article supporting the practice. Clark et al prospectively followed 188 women for lymphedema and measured them at baseline, 6 months, and 3 years after axillary surgery. At 3 years, patients were also questioned about skin punctures. Overall, 39 (21%) had lymphedema at 3 years.[35] Among other risk factors, univariate analysis found skin puncture for IV catheter insertion, venipuncture for blood draw, or finger stick for blood glucose testing were associated with a significant increased risk for lymphedema, with a risk ratio of 2.44 (95% CI, 1.33–4.47). These data should be interpreted cautiously; 18 of 188 (9.5%) reported remembering any type of skin puncture, and of these 18 women only 8 had lymphedema at the 3-year follow-up. Patients with lymphedema may be more likely to recall previous skin puncture than those without symptoms.
Hand surgery ipsilateral to the side of prior axillary surgery could be considered a severe form of skin puncture, lending relevance to the IV and venipuncture question. A small number of studies in the hand surgery literature address this occurrence. Dawson et al reported on 15 women undergoing carpel tunnel release ipsilateral to the side of prior axillary dissection; none had a post–hand surgery infection, new onset of lymphedema, or worsening of existing lymphedema symptoms.[36] In a second series, Hershko and Stahl retrospectively reviewed all hand surgeries performed between 1983 and 2002 and identified only 25 women with 1-year follow-up, of whom 4 had lymphedema at the time of hand surgery and 21 did not. Lymphedema was determined by patient perception only and noted to be worse after surgery in 2 women, each of whom had pre-existing lymphedema. The authors noted no progression or new symptoms in the remaining 23 women. Interestingly, they did not use preoperative antibiotics on any patient, performed 24 of 25 surgeries under local anesthesia, and used a tourniquet in all cases.[37] Gharbaoui et al surveyed the members of the American Society for Surgery of the Hand.[38] Among the 606 surgeons returning the surveys, more than 95% said they have willingly performed hand surgery on patients with a history of ipsilateral lymphadenectomy, radiation, or both. Surgeon willingness to operate decreases if lymphedema is present but remains quite high at 85%. Overall, 94% and 74% of surgeons routinely use tourniquets in women without and with lymphedema, respectively. Surgeon recollection identified fewer complications of infection, lymphedema, or delayed wound healing among patients without lymphedema (3% vs 23%). These three studies offer marginal data and anecdotal reports at best about the safety of ipsilateral skin puncture as they are all retrospective with small numbers, are fraught with recall bias, and lack any objective measurements. Despite this, each study calls for re-evaluation of the skin puncture guidelines as they relate to lymphedema risk. Perhaps the authors should also conclude that re-evaluation of the ipsilateral blood pressure guidelines is warranted, as their data demonstrated a clear willingness to use tourniquets with minimal reported morbidity. No documented studies to date have reviewed the recommendation to avoid ipsilateral blood pressure measurements. Clearly, these conclusions must be interpreted with caution, and further study is warranted. While the recommendations to wear compression garments while flying, and to avoid IV sticks, venipuncture, and blood pressures are made with good intention and based on sound physiologic principles, few rigorous data support them. Unfortunately, though, without good scientific data supporting or refuting these practices, both clinicians and patients are still faced with the clinical dilemma of whether or not they should encourage or disregard these behaviors.
Finally, exercise has historically been discouraged for breast cancer survivors, based on the belief that it would increase metabolic waste and extracellular fluid accumulation in the extremity, causing lymphedema. Over the last 5 years, the recommendation to avoid exercise has been challenged. Aside from recommended flexibility and remedial exercises used to reduce arm swelling and promote mobility during lymphedema treatment, exercise consists of resistance exercises (weight lifting), aerobic exercise, or a combination of both. Robust data support the value of resistance exercise in both at-risk and affected women. In fact, five of six randomized controlled trials (RCTs) published between 2006 and 2010 found weight lifting to be associated with minimal risk of developing or exacerbating lymphedema.[39] The largest study with the longest follow-up is the physical activity and lymphedema trial (PAL) that followed 141 women with breast cancer–related lymphedema. Patients were randomized to either weight training while wearing a compression sleeve, which was supervised twice weekly, or to a control group who were asked not to alter their exercise level. At 1-year follow-up, the authors found no increase in lymphedema in the intervention group and, further, found fewer and less severe lymphedema exacerbations in the weight training group.[40] The follow-up study evaluating weight training in at-risk survivors also found no difference in lymphedema rates between the two groups but interestingly found that among women with more than five nodes removed, those in the weight training group were significantly less likely to develop lymphedema than those in the control group (7% vs 22%, P = .003).[41]
Based on these data, the NLN now supports the safety of resistance exercises in a controlled manner, starting with small weights, low repetition, and gradual progression.[12] In general, compression garments should be worn during exercise in women with lymphedema and considered on an individual basis for women at risk for lymphedema.
A recent meta-analysis reviewed seven studies evaluating aerobic exercise in people with lymphedema.[39] Among the three randomized controlled trials and the four literature reviews, aerobic exercise or the combination of resistance and aerobic exercise did not trigger lymphedema or increase its incidence. Despite the relative agreement between the studies, the meta-analysis cautions that the benefits of aerobic exercise should be weighed against potential harms, as the data were not robust. Specifically, only one of three RCTs had more than 50 patients, but it was hampered by a trial adherence rate of only 70%.[42] Although aerobic exercises appear safe, additional well-designed studies with larger numbers and longer follow-up are needed.
Conclusions
As long as evaluation and treatment of the axilla are necessary, lymphedema will continue to be a potential morbidity. It is hoped that the move towards personalized medicine, by individualizing surgery, radiation, and chemotherapy regimens, will help to reduce the incidence of treatment morbidities, including lymphedema. Until the risk of lymphedema is eliminated, however, risk-reduction practices will remain a consideration for all breast cancer survivors. Clinicians should recognize that patients are not at equal risk for lymphedema and that data do not support a standardized approach nor allow for complete disregard of risk-reduction behaviors. Exercise in a monitored fashion is likely to be safe in all survivors, and the recommendation not to exercise appears to be the only myth surrounding lymphedema that has been scientifically disproven.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. National Cancer Institute: SEER Stat Fact Sheets: All Sites. Available at http://seer.cancer.gov/statfacts/html/all.html#survival. [updated on Nov. 10, 2011]
2. Cormier JN, Askew RL, Mungovan KS, et al. Lymphedema beyond breast cancer. Cancer. 2010;
116:5138-49.
3. Shah C, Vicini FA. Breast cancer related arm lymphedema: incidence rates, diagnostic techniques, optimal management and risk reduction strategies. J Radiat Oncol Biol Phys. 2011;81:907-14.
4. Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390-7.
5. Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368-77.
6. Rockson SG. Lymphedema. Am J Med. 2001;
110:288-95.
7. McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26:5213-9.
8. Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959-72.
9. McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in 936 women with breast cancer 5 years after sentinel node biopsy or axillary dissection: patient perceptions and precautionary behaviors. J Clin Oncol. 2008;26:5220-6.
10. Czerniec SA, Ward LC, Refshauge KM, et al. Assessment of breast cancer-related arm lymphedema-comparison of physical measurement methods and self report. Cancer Invest. 2010;28:54-62.
11. Cornish BH, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34:2-11.
12. National Lymphoma Network: NLN Position Papers 2011. Available at www.lymphnet.org.
13. Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg. 2004;187:69-72.
14. Hinrichs CS, Watroba NL, Rezaishiraz H, et al. Lymphedema secondary to postmastectomy radiation: incidence and risk factors. Ann Surg Oncol. 2004;
11:573-80.
15. Herd-Smith A, Russo A, Muraca MG, et al. Prognostic factors for lymphedema after primary treatment of breast carcinoma. Cancer. 2001;92:1783-7.
16. Kiel KD, Rademacker AW. Early-stage breast cancer: arm edema after wide excision and breast irradiation. Radiology 1996;198:279-83.
17. Paskett ED, Naughton MJ, McCoy TP, et al. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16:775-82.
18. Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546-53.
19. Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2003;23:4312-21.
20. Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP-B32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102:111-18.
21. Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657-63.
22. Goldberg JL, Wiechmann LL, Riedel ER, et al. Morbidity of sentinel node biopsy in breast cancer: the relationship between the number of excised lymph nodes and lymphedema. Ann Surg Oncol. 2010;17:3278-86.
23. Kwan W, Jackson J, Weir LM, et al. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol. 2002;20:4242-8.
24. Coen JJ, Taghian AG, Kachnic LA, et al. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. Int J Radiat Oncol Biol Phys. 2003;55:1209-15.
25. Norman SA, Localio AR, Kallan MJ, et al. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev. 2010;19:2734-46.
26. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569-75.
27. Reznik J, Cicchetti MG, Degaspe B, et al. Analysis of axillary coverage during tangential radiation therapy to the breast. Int J Radiat Oncol Biol Phys. 2005;61:163-8.
28. National Comprehensive Cancer Network: Lymphedema Guidelines. Available at www.NCCN.com.
29. National Cancer Institute: Lymphedema (PDQ). Available at http://www.cancer.gov/cancertopics/pdq/supportivecare/lymphedema/healthprofessional.
30. Boccardo FM, Ansaldi F, Bellini C, et al. Prospective evaluation of a prevention protocol for lymphedema following surgery for breast cancer. Lymphology. 2009;42:1-9.
31. Lee TS, Kilbreath SL, Sullivan G, et al. Factors that affect intention to avoid strenuous arm activity after breast cancer surgery. Oncol Nurs Forum. 2009;
36:454-62.
32. Casley-Smith JR. Lymphedema initiated by aircraft flights. Aviat Space Environ Med. 1996;67:52-6.
33. Graham PH. Compression prophylaxis may increase the potential for flight-associated lymphoedema after breast cancer treatment. Breast. 2002;11:66-70.
34. Kilbreath SL, Ward LC, Lane K, et al. Effect of air travel on lymphedema risk in women with history of breast cancer. Breast Cancer Res Treat. 2010;120:649-54.
35. Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. Q J Med. 2005;98:343-8.
36. Dawson WJ, Elenz DR, Winchester DP, et al. Elective hand surgery in the breast cancer patient with prior ipsilateral axillary dissection. Ann Surg Oncol. 1995;2:132-7.
37. Hershko DD, Stahl S. Safety of elective hand surgery following axillary lymph node dissection for breast cancer. Breast J. 2007;13:287-90.
38. Gharbaoui IS, Netscher DT, Thornby J, et al. Safety of upper extremity surgery after prior treatment for ipsilateral breast cancer: results of an American Society for Surgery of the Hand membership survey and literature review. J Am Soc Surg Hand. 2005;
5:232-8.
39. Kwan ML, Cohn JC, Armer JM, et al. Exercise in patients with lymphedema: a systematic review of the contemporary literature. J Cancer Surviv. 2011;5:320-6.
40. Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664-73.
41. Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer related lymphedema: a randomized trial. JAMA. 2010;304:2699-705.
42. Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396-404.