Lymphoma 2006: Classification and Treatment
The past 20 years have brought significant advances in our ability to manage patients with non-Hodgkin's lymphoma. More precise classification systems, improvements in diagnosis and staging, and effective new treatments have improved outcomes and made cure a reasonable goal for many patients with these disorders.
The past 20 years have brought significant advances in our ability to manage patients with non-Hodgkin's lymphoma. More precise classification systems, improvements in diagnosis and staging, and effective new treatments have improved outcomes and made cure a reasonable goal for many patients with these disorders. In this overview of the progress seen in the field over the past 2 decades, we describe a variety of advances for specific lymphomas, including diagnostic methods such as gene array studies and immunophenotyping and new treatment approaches.
Advances in understanding and treating hematologic malignancies have provided a paradigm for advances in other cancers. Over the past 20 years, we have seen important advances in the classification, diagnosis, staging, prognosis, and treatment of patients with lymphomas.
Historical Background
Before reviewing the current status of our understanding of lymphomas and our ability to manage patients with these disorders, it is instructive to remember the situation in this field 20 years ago. The Working Formulation for classifying non-Hodgkin's lymphomas was published in 1982[1] and was replacing the Rappaport classification[2] and Lukes-Collins classification[3] in the United States. Although the Working Formulation was a melange of the concepts and terms of the Rappaport classification, Lukes-Collins classification, and Kiel classification,[4] it was never adopted as widely in Europe as in North America. The Kiel classification developed by Dr. Karl Lennert and colleagues remained widely used throughout Europe. However, the fact that the major journals in hematology/oncology demanded the use of the Working Formulation in publications eventually led to its predominance in published research.
In 1986, the diagnosis of subtypes of non-Hodgkin's lymphomas was not nearly as accurate as most clinicians believed.[5-8] A high degree of inter- and intraobserver variability was found in studies of pathologists' ability to make reproducible diagnoses. The chance that the specific subtype of non-Hodgkin's lymphoma could be reproduced by another pathologist was not much more than 50%. This obviously had huge implications for interpreting clinical publications. This state of affairs probably reflected the lack of widespread use of sophisticated immunophenotyping, frequent lack of access to cytogenetics, and the diverse definitions of lymphoma subtypes that were in use.
In 1986, the Ann Arbor Staging system[9] was the most widely utilized approach to categorizing patients for risk and for choosing therapy. However, in that year, papers began to appear suggesting that it was possible to use other factors in addition to the Ann Arbor system to better predict outcome and select patients for therapy.[10,11] While computed tomography (CT) scans were widely utilized, postiron-emission tomography (PET) scans had not been introduced, and gallium scanning-the most widely available functional imaging method-proved problematic in other than the most experienced hands. Although still widely used in Hodgkin's disease, staging laparotomies were much less often used in staging patients with non-Hodgkin's lymphoma.
The optimal treatment for patients with "diffuse large cell lymphoma" was a point for debate in 1986. The CHOP regimen (cyclophosphamide, doxorubicin HCl, vincristine [Oncovin], prednisone) had been described a decade earlier, and this therapy or its variants were widely used. However, several new "third-generation" regimens including m-BACOD (methotrexate, bleomycin, doxorubicin [Adriamycin], cyclophosphamide, Oncovin, dexamethasone), ProMACE-MOPP (prednisone, methotrexate, Adriamycin, cyclophosphamide, etoposide, mechlorethamine, Oncovin, procarbazine [Matulane], prednisone), and MACOP-B (methotrexate, Adriamycin, cyclophosphamide, Oncovin, prednisone, bleomycin) were widely suspected of being superior to CHOP.
Patients with follicular lymphoma and those with small lymphocytic lymphoma were often treated initially with chlorambucil (Leukeran). The very intensive programs that are used today to treat Burkitt's lymphoma and lymphoblastic lymphoma were widely utilized in pediatrics but had not become as accepted in treating adults. Many of the subtypes of lymphoma that today provide a focus for therapeutic clinical research had not yet been described.
Classification of Non-Hodgkin's Lymphoma
In 1994, a group of hematopathologists from Europe and America proposed a new approach for classifying non-Hodgkin's lymphomas.[12] Rather than basing such classifications solely on the histopathologic characteristics of the tumor cells (ie, size, shape, and growth pattern), the authors proposed that immunologic characteristics, genetic characteristics, and the clinical characteristics of the disorders should all be taken into account in trying to identify homogeneous clinical pathologic entities.
The concepts in this Revised European-American Classification of Lymphoid Neoplasms (REAL) classification were tested in an international study of more than 1,400 cases from eight countries and four continents.[13] Five expert hematopathologists were able to diagnose lymphomas much more reproducibly than had been found in tests of previous systems, and the new clinical entities proposed in the REAL classification were distinctive. This approach was subsequently adopted as the World Health Organization (WHO) classification[14] and has become the standard approach for clinicians and investigators worldwide (Table 1).
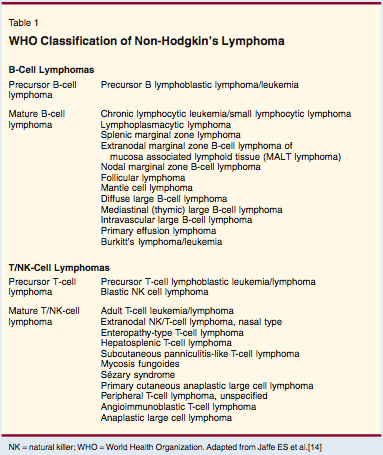
Despite the advances embodied in the WHO classification, this is clearly not the last word. As has been true in the past, our knowledge of the biology of lymphomas continues to advance, and these new insights can lead to the recognition of clinically important subgroups of lymphomas that have not been previously known. The REAL and WHO classifications came into being in part because of earlier observations that led to descriptions of mucosa-associated lymphoid tissue (MALT) lymphoma, anaplastic large T/null cell lymphoma, and mantle cell lymphoma-subtypes that were not found in previous classifications.
Gene Array Studies
In recent years, the application of gene array technology has provided fascinating new insights into non-Hodgkin's lymphomas. Gene expression patterns have enabled the identification of two, and probably more, distinct subsets of diffuse large B-cell lymphoma.[15,16] Patients with a subtype termed germinal center type have twice the survival of another group with the activated B-cell subtype-at least when the same treatments are utilized (Figure 1). These different subtypes of diffuse large B-cell lymphoma cannot be distinguished morphologically.
Diffuse large B-cell lymphoma occurring in the mediastinum is clinically distinctive and associated with younger age and a female predominance that differs from diffuse large B-cell lymphoma involving other sites. Gene array studies have shown that this lymphoma is also distinctive in its gene expression pattern[17] and actually has a gene expression pattern more similar to Hodgkin's disease than to other diffuse large B-cell lymphomas.
Distinguishing between Burkitt's lymphoma and diffuse large B-cell lymphoma can sometimes be problematic. The strikingly different gene expression patterns seen in the two disorders allow this distinction to be made more accurately than can be done morphologically and can be used to guide therapy.[18] At least one study has found that the outcome of patients with follicular lymphoma might be better predicted by the gene expression pattern in the "normal" immune cells in the tumor than by the pattern in the tumor cells themselves.[19]
Diagnosis of Non-Hodgkin's Lymphoma
The diagnosis of a non-Hodgkin's lymphoma is best made by an experienced hematopathologist working with an excisional biopsy and with fresh tissue frozen for specialized studies if needed. Although there has been enthusiasm in some quarters for the use of fine-needle aspirates to diagnose non-Hodgkin's lymphomas, this technique is better reserved for documenting relapse at a site difficult to biopsy in a patient with a known type of non-Hodgkin's lymphoma.
Fine-Needle Aspiration
Hehn and colleagues studied the use of fine-needle aspiration in 93 patients as the initial diagnostic study for lymphoma.[20] A specific and complete histologic diagnosis using an accepted classification system (Working Formulation, REAL, or WHO) was possible in only 27 patients (29%).[20] Sixty-seven patients had both excisional biopsies and fine-needle aspirates available for study. When immunophenotyping was completed on the specimen obtained by fine-needle aspirate, 29% of those cases yielded the same diagnoses as an excisional biopsy. When complete immunophenotyping was not available on the material obtained by fine-needle aspiration, only 2% of the cases yielded the same diagnosis as was found on the excisional biopsy. The discrepancies included several circumstances that would have led to inappropriate therapy if the therapeutic decision was based on the fine-needle aspirate.
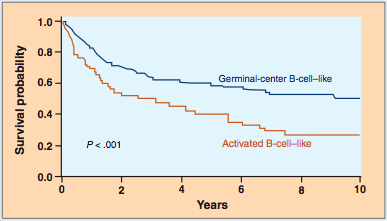
Figure 1: Overall Survival According to Risk Group--Note difference in survival for patients with a diffuse large B-cell lymphoma that had a gene expression pattern similar to normal germinal center B-cells vs those whose tumors expressed genes similar to activated B cells. Adapted from Rosenwald A et al.[16]
Langren and colleagues[21] reported 103 patients who underwent both fine-needle aspirate and excisional biopsy. Although they found a higher degree of concordance, they pointed out that the use of fine-needle aspiration as a standard biopsy technique for non-Hodgkin's lymphoma would lead to loss of archival tissue for complimentary analyses, reclassification, and research purposes. In addition, they found that T-cell lymphomas could not be accurately diagnosed utilizing the needle aspiration technique.
Immunophenotyping
Immunophenotyping has made an important contribution to the diagnosis of non-Hodgkin's lymphomas. While some histopathologic subtypes can be accurately diagnosed based on morphology alone (eg, follicular lymphoma), immunophenotyping is important for most patients .
An international study of 1,403 cases of non-Hodgkin's lymphoma involved five expert hematopathologists who independently made diagnoses on each case both before and after immunophenotyping.[13] Follicular lymphoma was reproducibly diagnosed 93% of the time without and 94% of the time with immunophenotyping. However, the accuracy of grading follicular lymphomas varied from 60% to 72% and was not improved by immunophenotyping. Diffuse large B-cell lymphoma was reproducibly diagnosed 73% of the time before and 87% of the time after immunophenotyping. Anaplastic large T/null cell lymphoma and other peripheral T-cell lymphomas could be reproducibly diagnosed only 41% to 46% of the time before immunophenotyping but 85% to 86% of the time after immunophenotyping.
Anaplastic large T/null cell lymphoma frequently overexpresses the ALK protein. This can be identified by immunophenotyping, and the presence or absence of this protein has a significant impact on prognosis, with patients who are ALK-positive having superior survival to those who are ALK-negative.[22] The diagnosis of non-Hodgkin's lymphoma should always be made in a setting where immunophenotyping is available.
Genetic Studies
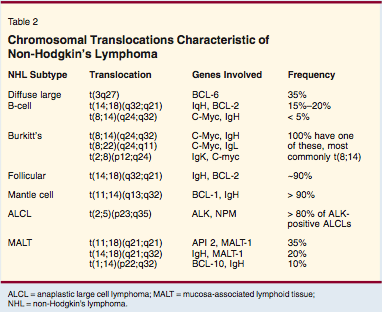
The use of genetic studies to diagnose non-Hodgkin's lymphomas is becoming increasingly important. Several genetic abnormalities (that are characteristic of specific subtypes of non-Hodgkin's lymphoma) might be identified by cytogenetics or by fluorescence in situ hybridization (FISH) studies, which can resolve diagnostic dilemmas (Table 2). In addition, gene array studies have shown the potential for improving diagnostic accuracy. For example, Dave and colleagues have shown that difficulties in distinguishing between diffuse large B-cell lymphoma and Burkitt's lymphoma can be accurately resolved by the use of gene arrays.[18] It is likely that gene array studies will become commercially available in the future and make an important contribution to our ability to diagnose patients with non-Hodgkin's lymphoma.
Staging/Prognostic Systems for Non-Hodgkin's Lymphoma
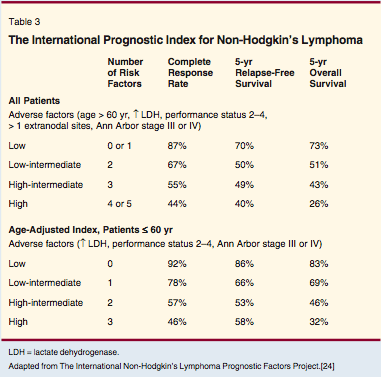
The sixth edition of the AJCC Cancer Staging Manual[23] recommends anatomic staging using the Ann Arbor Staging System and adopts the International Prognostic Index[24] as a useful tool in making treatment decisions. The International Prognostic Index (Table 3) takes into account anatomic stage, age, performance status, serum lactate dehydrogenase level, and the presence or absence of multiple extranodal sites of lymphoma. This is easily used in the clinic, as the score is the addition of the five adverse risk factors (ie, total score of 0 to 5), and can be remembered easily with the acronym "APLES." When applied to patients with aggressive non-Hodgkin's lymphomas, the International Prognostic Index improved the ability to predict outcome over anatomic staging alone. Patients with Ann Arbor stage II had 5-year survival rates ranging from 40% to 75% depending on their International Prognostic Index score, and patients with Ann Arbor stage IV had survival rates ranging from 25% to 65%.
The International Prognostic Index can be applied to all subtypes of non-Hodgkin's lymphoma. However, it is clearly not the final answer to determining prognosis in patients with this disease, and alternate prognostic systems have been developed for follicular lymphoma[25] and peripheral T-cell lymphoma.[26] In addition, new treatments (eg, rituximab [Rituxan]), and new biologic measurements (eg, gene arrays) are almost certain to modify some aspects of the International Prognostic Index.[27,28] For example, a recent report showed that in patients with diffuse large B-cell lymphoma who received CHOP and rituximab, outcome was better predicted by a modification of the International Prognostic Index.[28] The ability to apply staging criteria by tightly defining the interpretation of test results has important implications for non-Hodgkin's lymphomas in general[29,30] and for specific sites of involvement such as primary central nervous system lymphoma.[31]
Advances in Treatment for Non-Hodgkin's Lymphoma
Diffuse Large B-Cell Lymphoma
The publication by Fisher et al in 1993[32] showing that CHOP therapy produced an equivalent survival to that found with more complicated and more toxic regimens made CHOP the standard regimen in North America for patients with disseminated diffuse large B-cell lymphoma. This changed in 2002, with a publication by Coiffier et al showing that in elderly patients, the combination of rituximab and CHOP was superior to CHOP alone.[33] Although the study involved only patients 60 years of age or older, this was quickly and widely applied to patients of all ages.
In France, CHOP has not been administered to young patients with diffuse large B-cell lymphoma. These patients have received ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone),[34] and a study is under way to see if the combination of rituximab plus ACVBP will be superior. In Germany, based on the results of a national trial, the standard therapy for patients over age 60 has been CHOP given at two weekly intervals and, for patients younger than 60, CHOP plus etoposide.[35] It has recently been shown that CHOP plus rituximab is equivalent to CHOP plus etoposide plus rituximab in young patients, and that CHOP at 14-day intervals plus rituximab is an effective therapy in patients over 60,[36] but not necessarily better than CHOP plus rituximab administered at 21-day intervals.
An international study of a "CHOP-like" regimen with or without rituximab in younger, good-prognosis patients with diffuse large B-cell lymphoma confirmed the superiority of rituximab-containing regimens in patients with diffuse large B-cell lymphoma.[37] In addition, Sehn and colleagues looked at the impact of ritixumab in addition to CHOP on a population basis in British Columbia.[38] Because slides are centrally reviewed, anticancer drugs are dispensed from a single pharmacy, and the availability of rituximab depended on a government decision in British Columbia, a unique opportunity presented itself to observe the impact of the agent on a province-wide basis. Patients who were treated 18 months before and 18 months after the introduction of rituximab were evaluated. With the only variable being the date that the drug became approved, and recognizing that not all patients received the drug after approval, the survival rate for patients with diffuse large B-cell lymphoma in British Columbia went up by approximately 20%, confirming the utility of this combination.
Patients with localized diffuse large B-cell lymphoma have a much better prognosis than patients with disseminated disease. In North America, the standard treatment for these patients has been three or four cycles of CHOP followed by involved-field radiotherapy, as described by Miller and colleagues in 1998.[39] However, French investigators have recently found that ACVBP alone is superior to CHOP plus radiotherapy in younger patients with localized aggressive lymphoma.[40] In another study in older patients, the same investigators found that CHOP alone for four cycles was equivalent to CHOP plus radiotherapy in patients over 60 years of age, and that the radiotherapy-containing arm might actually be inferior in patients over age 70.[41]
The impact of rituximab in addition to CHOP or another chemotherapy regimen in patients with localized disease is not yet clear. The Southwest Oncology Group compared the results of a phase II trial of CHOP plus rituximab for four cycles followed by involved-field radiotherapy with historical controls who did not receive rituximab and showed a lower failure rate with the addition of the antibody.[42]
Other treatment approaches have been tested in patients with diffuse large B-cell lymphoma, including early autologous bone marrow transplantation in high-risk patients. While the results of many studies have been sometimes confusing and contradictory, they at least hint that a subset of patients might benefit from adjuvant high-dose therapy and autologous bone marrow transplantation.[43]
Follicular Lymphoma
Today, the initial treatment approach for patients with follicular lymphoma might entail observation, single-agent chemotherapy, CVP (cyclophosphamide, vincristine, prednisone), CHOP, fludarabine-containing regimens, rituximab, radiolabeled antibodies, or combinations of these strategies. Whenever such a wide variety of treatment choices are in use, it is clear that there is no proven best approach.
Observation without therapy for asymptomatic patients was proposed many years ago by investigators from Stanford[44] and remains a viable option. For patients who are asymptomatic without organ compromise and who have either localized or disseminated disease, it appears that initial observation does not worsen the ultimate outcome.
When patients do require therapy, the multitude of effective treatments makes a decision difficult for oncologists. While there are strong advocates for each treatment approach, it is not yet clear that one strategy is superior. Important ongoing trials such as the US trial comparing CHOP plus rituximab vs CHOP followed by the radiolabeled monoclonal antibody I131 tositumomab (Bexxar) might eventually change this situation. Until then, recent studies have suggested certain principles on which treatment decisions can be based.
When patients are treated initially with single-agent rituximab, it is now clear that ongoing or "maintenance" treatment with the antibody will lengthen remissions.[45,46] Whether ongoing rituximab is best administered by one dose every other month, reinduction with four weekly doses every 6 months, or another approach is unclear. At least one study has shown that the radiolabeled antibody I-131 tositumomab has an extremely high remission rate and yields prolonged remissions when used as the initial therapy, but this approach is rarely reimbursed by insurers or Medicare in the United States.[47]
While the best combination chemotherapy regimen for follicular lymphoma remains a point of debate, it is increasingly clear that the addition of rituximab to any of the popular regimens increases the response rate and prolongs remission duration. Recent studies have found an apparent increase in survival associated with the addition of rituximab.[48-50] When induction therapy does not include rituximab, ongoing or "maintenance" rituximab prolongs remission duration. Whether this applies to patients who receive rituximab with their induction treatment is less clear.
Patients with follicular lymphoma who relapse can have long remissions after both autologous and allogeneic bone marrow transplantation. Our results, and those reported from St. Bartholomew's Hospital in London[51] have found a 40% to 50% 10-year remission rate in patients who were transplanted at first treatment failure. A recent study from Germany suggests that first-remission autotransplants might improve outcomes over "standard" treatments.[52] A significant problem in these patients undergoing autologous transplantation continues to be the development of myelodysplasia. Allogeneic transplantation appears to be potentially curative in relapsed follicular lymphoma.[53] The higher treatment-related mortality rate has made it difficult to show an advantage of allogeneic over autologous transplantation, but reduced-intensity allotransplants appear to reduce treatment-related mortality.[54]
Mantle Cell Lymphoma
Mantle cell lymphoma has been among the most difficult non-Hodgkin's lymphomas to treat. Utilizing standard chemotherapy regimens, the remission duration has been brief and overall survival on the average of 3 to 4 years. The very intensive hyper-CVAD regimen (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) seems to improve response rate and remission duration and has been widely adopted for treating patients who are young enough to tolerate this approach.[55] The addition of rituximab to hyper-CVAD seems to further improve treatment results.[56] However, rituximab plus CHOP is also superior to CHOP alone,[57] and whether or not the more intensive regimen is superior has not been proven in a prospective trial.
Patients with mantle cell lymphoma often undergo autologous, or rarely allogeneic, hematopoietic stem cell transplantation in first remission. While this treatment approach is widely utilized because of the poor outcome seen with less intensive treatments, only one prospective clinical trial has been published showing a superior outcome of patients undergoing stem cell transplant.[58]
Burkitt's Lymphoma
More than 20 years ago, Burkitt's lymphoma in adults had a dismal prognosis, with most patients dying within a year of diagnosis. However, the adoption of very intensive chemotherapy regimens similar to those used in pediatric patients has changed this outcome. The CODOX-M regimen (cyclophosphamide, vincristine, doxo-
rubicin, high-dose methotrexate) developed by Magrath and colleagues,[59] and the hyper-CVAD regimen from M. D. Anderson have both shown that it is possible to cure the majority of adults with Burkitt's lymphoma. It appears that the addition of rituximab to either regimen is useful.[60] There does not appear to be a place for hematopoietic stem cell transplantation in the initial treatment of patients with Burkitt's lymphoma, and patients who relapse are rarely salvaged with any approach.
MALT Lymphoma
Twenty years ago, the lymphomas that are known collectively as marginal zone lymphomas (ie, MALT, nodal marginal zone, and splenic marginal zone) were unknown to most oncologists, although Peter Isaacson had described MALT lymphomas in 1983.[61] Most cases of what today would be called MALT lymphoma were classified as small lymphocytic lymphoma, another subtype of small cell non-Hodgkin's lymphoma, or even as pseudolymphoma. The demonstration that gastric MALT lymphomas were associated with Helicobacter pylori infection and frequently regressed with eradication of the H pylori led to wide acceptance of this concept.[62]
Today, the initial treatment for gastric MALT lymphomas in patients who are H pylori-positive in whom the lymphoma has not metastasized or deeply invaded the stomach and lacks adverse chromosomal translocations remains H pylori eradication and close follow-up. It appears that many if not most of these patients remain polymerase chain reaction (PCR)-positive for the lymphoma, but prolonged remissions have been observed and not all patients relapse.[63]
Patients who do not respond to eradication of the H pylori (or are not infected) frequently have long-term remissions induced by radiotherapy. Gastric resection is not an appropriate therapy for this lymphoma. However, numerous systemic therapies are effective including alkylating agents such as chlorambucil and the antibody rituximab. Patients with localized MALT lymphomas occurring in sites other than the stomach can sometimes have long remissions induced by resection or involved-field radiotherapy. Single-agent or combination chemotherapy and rituximab are also effective approaches.
Peripheral T-Cell Lymphoma
Peripheral, or mature, T-cell lymphomas represent a complex group of neoplasms that have been, for the most part, poorly studied to date. The WHO classification includes a variety of distinctive clinical pathologic syndromes that can be divided into lymphomas that usually originate in lymph nodes and those that usually originate in extranodal sites (Table 4). In general, patients with the peripheral T-cell lymphomas have a chance for long-term survival that is approximately one-half that seen with diffuse large Bcell lymphoma.[13,64] This undoubtedly reflects the fact that treatment regimens in use today were developed in studies where the largest number of patients studied had diffuse large Bcell lymphoma. Until studies focusing primarily on peripheral T-cell lymphomas identify the most active drugs and combinations for these disorders, the situation is not likely to change.
A recent large international study confirmed the utility of the WHO subtypes of peripheral T-cell lymphoma and the poor prognosis that has been generally reported.[65] The one exception to the poor outlook associated with peripheral T-cell lymphoma, seems to be patients with anaplastic large T/null cell lymphomas whose tumors overexpress the ALK protein.[22] These patients respond to anthracycline-containing chemotherapy regimens and have a survival comparable to or better than that seen with aggressive B-cell lymphoma.[66,67] Hopefully, improved treatments for the other patients with peripheral
T-cell lymphomas will be the result of clinical trials focusing on these disorders.
Conclusions
Over the past 28 years we have seen a revolution in our ability to manage patients with non-Hodgkin's lymphoma. Better and more precise classification systems that allow patients with uniform diseases to be studied, improvements in diagnosis and staging, and new treatments have improved the overall outcome and made cure at least a reasonable goal for many patients with these disorders. Hopefully, the next 20 years will bring us equal or greater advances.
Disclosures:
Dr. Armitage is a lecturer for Roche, and a consultant and lecturer for GlaxoSmithKline and Biogen IDEC.
References:
1. National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas: Summary and description of a working formulation for clinical usage. The Non-Hodgkin’s Lymphoma Pathologic Classification Project. Cancer 49:2112-2135, 1982.
2. Hicks EB, Rappaport H, Winter WJ: Follicular lymphoma: A re-evaluation of its position in the scheme of malignant lymphoma, based on a survey of 253 cases. Cancer 9:792-821, 1956.
3. Lukes RJ, Collins RD: Immunologic characterization of human malignant lymphomas. Cancer 34(4 suppl):1488-503, 1974.
4. Lennert K, Stein H, Kaiserling E: Cytological and functional criteria for the classification of malignant lymphomata. Br J Cancer 31(suppl 2):29-43, 1975.
5. Kim H, Zelman RJ, Fox MA, et al: Pathology Panel for Lymphoma Clinical Studies: A comprehensive analysis of cases accumulated since its inception. J Natl Cancer Inst 68:43-67, 1982.
6. Whitcomb CC, Crissman JD, Flint A, et al: Reproducibility in morphologic classification of non-Hodgkin’s lymphomas using the Lukes-Collins system. The Southeastern Cancer Study Group experience. Am J Clin Pathol 82:383-388, 1984.
7. Classification of non-Hodgkin’s lymphomas. Reproducibility of major classification systems. NCI non-Hodgkin’s Classification Project Writing Committee. Cancer 55:91-95, 1985.
8. Dick F, VanLier S, Banks P, et al: Use of the working formulation for non-Hodgkin’s lymphoma in epidemiologic studies: Agreement between reported diagnoses and a panel of experienced pathologists. J Natl Cancer Inst 78:1137-1144, 1987.
9. Carbone PP, Kaplan HS, Musshoff K, et al: Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res 31:1860-1861, 1971.
10. Shipp MA, Harrington DP, Klatt MM, et al: Identification of major prognostic subgroups of patients with large-cell lymphoma treated with m-BACOD or M-BACOD. Ann Intern Med 104:757-765, 1986.
11. Jagannath S, Velasquez WS, Tucker SL, et al: Tumor burden assessment and its implication for a prognostic model in advanced diffuse large-cell lymphoma. J Clin Oncol 4:859-865, 1986.
12. Harris NL, Jaffe ES, Stein H, et al: A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group [see comments]. Blood 84:1361-1392, 1994.
13. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 89:3909-3918, 1997.
14. Pathology and genetics of tumours of haematopoietic and lymphoid tissues, in Jaffe E, Harris N, Stein H, et al (eds): World Health Organization Classification of Tumors. Lyon, IARC Press, 2001.
15. Alizadeh AA, Eisen MB, Davis RE, et al: Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511, 2000.
16. Rosenwald A, Wright G, Chan WC, et al: The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346:1937-1947, 2002.
17. Savage KJ, Monti S, Kutok JL, et al: The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood 102:3871-3879, 2003.
18. Dave SS, Fu K, Wright G, et al: Gene expression distinguishes Burkitt lymphoma from other aggressive lymphomas and identifies patients who are highly curable with intensive chemotherapeutic regimens (abstract 415). Blood 106:125a, 2005.
19. Dave SS, Wright G, Tan B, et al: Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med 351:2159-2169, 2004.
20. Hehn ST, Grogan TM, Miller TP: Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol 22:3046-3052, 2004.
21. Landgren O, Porwit MacDonald A, Tani E, et al: A prospective comparison of fine-needle aspiration cytology and histopathology in the diagnosis and classification of lymphomas. Hematol J 5:69-76, 2004.
22. Gascoyne RD, Aoun P, Wu D, et al: Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood 93:3913-3921, 1999.
23. AJCC Cancer Staging Manual, 6th ed. New York, Springer, 2002.
24. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project: A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 329:987-994, 1993.
25. Solal-Celigny P, Roy P, Colombat P, et al: Follicular lymphoma International Prognostic Index. Blood 104:1258-1265, 2004.
26. Gallamini A, Stelitano C, Calvi R, et al: Peripheral T-cell lymphoma unspecified (PTCL-U): A new prognostic model from a retrospective multicentric clinical study. Blood 103:2474-2479, 2004.
27. Kersten MJ, Jong DdD, Raemaekers JM, et al: Beyond the International Prognostic Index: New prognostic factors in follicular lymphoma and diffuse large-cell lymphoma: A meeting report of the Second International Lunenburg Lymphoma Workshop. Hematol J 5:202-208, 2004.
28. Sehn LH, Chhanabhai M, Fitzgerald C, et al: Revised International Prognostic Index (R-IPI) is a better predictor ofoutcome than the standard IPI for patients with diffuse large B-cell lymphoma (DLBCL) treated with rituximab and CHOP (R-CHOP) (abstract 492). Blood 106:147a, 2005.
29. Tsimberidou AM, Sarris AH, Medeiros LJ, et al: Hodgkin’s disease in patients infected with human immunodeficiency virus: Frequency, presentation and clinical outcome. Leuk Lymphoma 41:535-544, 2001.
30. Cheson BD, Pfistner B, Juweid ME, et al: Revised response criteria for malignant lymphomas. From the members of the International Harmonization Project (IHP) of the competence network malignant lymphoma (abstract 18). Blood 106:10a, 2005.
31. Abrey LE, Batchelor TT, Ferreri AJ, et al: Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23:5034-5043, 2005.
32. Fisher RI, Gaynor ER, Dahlberg S, et al: Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma [see comments]. N Engl J Med 328:1002-1006, 1993.
33. Coiffier B, Lepage E, Briere J, et al: CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235-242, 2002.
34. Coiffier B, Reyes F: Best treatment of aggressive non-Hodgkin’s lymphoma: A French perspective. Oncology 19(suppl 1):7-15, 2005.
35. Pfreundschuh M, Trumper L, Kloess M, et al: Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood 104:634-641, 2004.
36. Pfreundschuch M, Kloess M, Schmits R, et al: Six, not eight cycles of Bi-weekly CHOP with rituximab (R-CHOP-14) is the preferred treatment for elderly patients with diffuse large B-cell lymphoma (DLBCL): Results of the RICOVER-60 trial of the German high-grade non-Hodgkin lymphoma study group (DSHNHL) (abstract 13). Blood 106:9a, 2005.
37. Pfreundschuch M, Truemper LGD, Gill D, et al: First analysis of the completed Mabthera International (MInT) Trial in young patients with low-risk diffuse large B-cell lymphoma (DLBCL): Addition of rituximab to a CHOP-like regimen significantly improves outcome of all patients with the identification of a very favorable subgroup with IPI=O and no bulky disease (abstract 157). Blood 104:48a, 2004.
38. Sehn LH, Donaldson J, Chhanabhai M, et al: Introduction of combined CHOP-rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma (DLBC) in British Columbia (BC) (abstract 88). Blood 102:29a, 2003.
39. Miller TP, Dahlberg S, Cassady JR, et al: Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma [see comments]. N Engl J Med 339:21-26, 1998.
40. Reyes F, Lepage E, Ganem G, et al: ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med 352:1197-1205, 2005.
41. Fillet G, Bonnet C, Mounier N, et al: No role for chemoradiotherapy when compared with chemotherapy alone in elderly patients with localized low risk aggressive lymhoma: final results of the LNH93-4 GELA study (abstract 15). Blood 106:9a, 2005.
42. Miller TP, Unger JM, Spier CM, et al: Effect of adding rituximab to three cycles of CHOP plus involved-field radiotherapy for limited-stage aggressive diffuse B-cell lymphoma (SWOG-0014) (abstract 158). Blood 104:48a, 2004.
43. Haioun C, Lepage E, Gisselbrecht C, et al: Survival benefit of high-dose therapy in poor-risk aggressive non- Hodgkin’s lymphoma: Final analysis of the prospective LNH87-2 protocol-a groupe d’Etude des lymphomes de l’Adulte study. J Clin Oncol 18:3025-3030, 2000.
44. Portlock CS, Rosenberg SA: No initial therapy for stage III and IV non-Hodgkin’s lymphomas of favorable histologic types. Ann Intern Med 90:10-13, 1979.
45. Hainsworth JD, Litchy S, Burris HA 3rd, et al: Rituximab as first-line and maintenance therapy for patients with indolent non-hodgkin’s lymphoma. J Clin Oncol 20:4261-4267, 2002.
46. Ghielmini M, Schmitz SF, Cogliatti SB, et al: Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 103:4416-4423, 2004.
47. Kaminski MS, Zasadny KR, Francis IR, et al: Radioimmunotherapy of B-cell lymphoma with [131I]anti-B1 (anti-CD20) antibody. N Engl J Med 329:459-465, 1993.
48. Hochster HS, Weller E, Gascoyne RD, et al: Maintenance rituximab after CVP results in superior clinical outcome in advanced follicular lymphoma (FL): Results of the E1496 phase III trial from the Eastern Cooperative Oncology Group and the Cancer and Leukemia Group B (abstract 349). Blood 106:106a, 2005.
49. Solal-Celigny P, Imrie K, Belch A, Robinson KS, et al: Mabthera (rituximab) plus CVP chemotherapy for first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma (NHL): Confirmed efficacy with longer follow-up (abstract #350). Blood 106:106a, 2005.
50. Schulz H, Skoetz N, Bohlius J, et al: Does combined immunochemotherapy with the monoclonal antibody rituximab improve overall survival in the treatment of patients with indolent non-Hodgkin’s lymphoma? Preliminary results of a comprehensive meta-analysis (abstract 351). Blood 106:106a, 2005.
51. Davies AJ, Apostolidis J, Micallef IN, et al: Long-term follow up of patients with follicular lymphoma (FL) receiving high dose therapy (HDT) with autologous haematopoietic progenitor cell support at St. Bartholomew’s Hospital (SBH) (abstract 6568). J Clin Oncol 23(16S):577s, 2005.
52. Lenz G, Dreyling M, Schiegnitz E, et al: Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: Results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood 104:2667-2674, 2004.
53. van Besien K, Loberiza FR Jr, Bajorunaite R, et al: Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood 102:3521-3529, 2003.
54. Escalon MP, Champlin RE, Saliba RM, et al: Nonmyeloablative allogeneic hematopoietic transplantation: A promising salvage therapy for patients with non-Hodgkin’s lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol 22:2419-2423, 2004.
55. Khouri IF, Romaguera J, Kantarjian H, et al: Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: An active regimen for aggressive mantle-cell lymphoma. J Clin Oncol 16:3803-3809, 1998.
56. Romaguera JE, Fayad L, Rodriguez MA, et al: High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol 23:7013-7023, 2005.
57. Lenz G, Dreyling M, Hoster E, et al: Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 23:1984-1992, 2005.
58. Dreyling M, Lenz G, Hoster E, et al: Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: Results of a prospective randomized trial of the European MCL Network. Blood 105:2677-2684, 2005.
59. Magrath I, Adde M, Shad A, et al: Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol 14:925-934, 1996.
60. Thomas D, Cortes J, Faderl S, et al: Outcome with the Hyper-CVAD and rituximab regimen in Burkitt (BL) and Burkitt-like (BLL) leukemia/lymphoma (abstract 3297). Blood 104:901a, 2004.
61. Isaacson P, Wright DH: Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer 52:1410-1416, 1983.
62. Wotherspoon AC, Doglioni C, Diss TC, et al: Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori [see comments]. Lancet 342:575-577, 1993.
63. Thiede C, Wundisch T, Alpen B, et al: Long-term persistence of monoclonal B cells after cure of Helicobacter pylori infection and complete histologic remission in gastric mucosa-associated lymphoid tissue B-cell lymphoma. J Clin Oncol 19:1600-1609, 2001.
64. Gisselbrecht C, Gaulard P, Lepage E, et al: Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood 92:76-82, 1998.
65. Vose J: International peripheral T-cell lymphoma (PTCL) clinical and pathologic review project: poor outcome by prognostic indices an lack of eficacy with anthracyclines (abstract 811). Blood 106:239a, 2005.
66. Ascani S, Zinzani PL, Gherlinzoni F, et al: Peripheral T-cell lymphomas. Clinico-pathologic study of 168 cases diagnosed according to the R.E.A.L. Classification. Ann Oncol 8:583-592, 1997.
67. Savage KJ, Chhanabhai M, Gascoyne RD, et al: Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol 15:1467-1475, 2004.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.