Management of Difficult Germ-Cell Tumors
Although testicular cancer is a rare disease accounting for only 1% of all male neoplasms, it represents a paradigm for cancer curability. Overall, more than 95% of patients can expect to be cured of their disease with minimal long-term toxicity. Given these expectations, it is critical that cancer care providers are familiar with the diagnostic and therapeutic challenges encountered in these rare patients. In particular, clinicians managing these patients should be aware of some of the pitfalls encountered when determining relapse. In a series of case presentations, we review the evaluation and management of patients with persistent elevation of serum tumor markers and postchemotherapy residual radiographic abnormalities.
Although testicular cancer is a rare disease accounting for only 1% of all male neoplasms, it represents a paradigm for cancer curability. Overall, more than 95% of patients can expect to be cured of their disease with minimal long-term toxicity. Given these expectations, it is critical that cancer care providers are familiar with the diagnostic and therapeutic challenges encountered in these rare patients. In particular, clinicians managing these patients should be aware of some of the pitfalls encountered when determining relapse. In a series of case presentations, we review the evaluation and management of patients with persistent elevation of serum tumor markers and postchemotherapy residual radiographic abnormalities.
Although an uncommon malignancy, testicular cancer provides a paradigm for the treatment of disease with the intent to cure. Indeed, more than 95% of men with testicular cancer are cured, with little long-term toxicity. Most testicular tumors are of germ-cell origin. The following three cases illustrate key considerations in the evaluation and management of patients with difficult germ-cell tumors.
Case 1
A healthy 25-year-old male was diagnosed with right testicular nonseminomatous germ-cell tumor with small-volume retroperitoneal metastases. Retroperitoneal lymph node dissection (RPLND) revealed 2 out of 13 positive nodes. Post-RPLND, the patient was followed with close surveillance. Three months later, human chorionic gonadotropin (HCG) levels rose precipitously and two pulmonary nodules were seen on chest imaging. Three cycles of bleomycin, etoposide, and cisplatin were administered, resulting in normalization of serum markers and radiographic abnormalities. Within 3 months of chemotherapy, HCG again rose to 1,006 ng/mL and a new pulmonary nodule was observed. Brain magnetic resonance imaging and testicular ultrasound ruled out disease recurrence in sanctuary sites. Salvage chemotherapy with vinblastine, ifosfamide, and cisplatin was initiated. After one cycle, the pulmonary nodule had enlarged (see Figure 1) and HCG continued to rise.
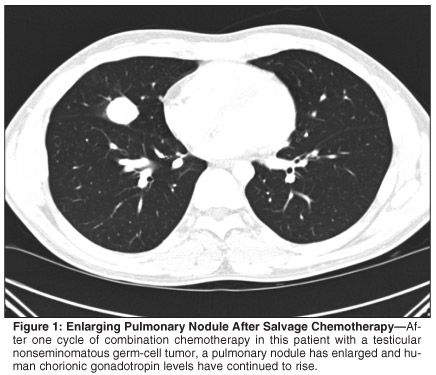
What is the appropriate intervention in this patient?
Germ-cell tumors are unique in that they remain highly curable even in the metastatic setting and, to a lesser degree, even after primary chemotherapy has failed. Standard-dose cisplatin-based chemotherapy regimens such as BEP (bleomycin, etoposide, cisplatin [Platinol]) and VIP (etoposide [VePesid], ifosfamide, cisplatin) result in cure in approximately 70% to 80% of patients.[1] Salvage chemotherapy with vinblastine, ifosfamide, and cisplatin is associated with complete response (CR) in 50% of patients, but this remission is durable in only 25%.[2]
Patients who experience disease progression within 4 weeks after cisplatin-based chemotherapy are described as having platinum-refractory disease, whereas absolute platinum-refractory disease is used to describe those patients whose disease progresses during cisplatin-based treatment.[3] The prognosis for this group of patients is particularly poor and has prompted the investigation of surgical salvage (occasionally referred to as "desperation surgery"). In the majority of cases, patients with persistently elevated serum markers have systemic disease that is not amenable to surgical intervention. However, an increasing body of data points to a definite cure rate for surgical salvage in a highly select group of patients with surgically resectable chemorefractory germ-cell tumors.
• Salvage Surgery in the Literature-Murphy et al[4] described 48 patients with chemorefractory disease who went on to surgical salvage. All patients had elevated serum markers at the time of surgery. Although 38 of 48 patients (79%) were rendered grossly disease-free after surgery, and 29 of 48 (60%) achieved serologic remission, only 10 of 48 (21%) remained continuously free of disease. Nineteen patients relapsed after obtaining remission with salvage surgery. Of these, four were rendered free of disease with further sugery, and two others obtained long-term remission after high-dose chemotherapy with autologous bone marrow transplant. Overall, 16 of 48 patients (33%) achieved long-term disease-free survival.
Ability to completely resect the disease was the factor most predictive of favorable outcome, and the authors emphasized that this group of patients made up only 8% of the 600 postchemotherapeutic resections performed at Indiana University over a 13-year period. Of 13 patients who had two or more sites of disease at the time of surgery, none were long-term survivors. Presurgical characteristics suggestive of favorable outcome included elevated alpha-fetoprotein (AFP) only at time of surgery, late relapse after chemotherapy, and presence of teratoma in the initial specimen.
This series confirmed many of the conclusions drawn by Wood et al[5] regarding the experience with surgical salvage at Memorial Hospital. Fifteen patients with chemorefractory disease and persistently elevated serum markers underwent surgical salvage. In each case, the entire residual mass or solitary metastasis was resected. Of these 15 patients, 12 were considered to have achieved CR with surgery as assessed by normalization of serum markers after resection. Seven of the patients who obtained CR with surgery alone remained without evidence of disease on follow-up 3 to 53 months after resection, but five relapsed within a median of 2 months after surgery. Two of the five relapsed patients achieved disease-free status with further chemotherapy. Of the three patients who did not achieve CR with surgery, two patients were rendered disease-free after postoperative salvage chemotherapy. Overall, 11 of the 15 patients (73%) were rendered disease-free after salvage surgery with or without additional therapy. Favorable prognostic factors in this cohort again included isolated AFP elevation and retroperitoneal site of disease.
Eastham and colleagues[6] reported their experience with surgical salvage in 16 patients with chemorefractory disease. Ten of these patients had a solitary metastasis in the retroperitoneum. Of this group, six patients had rising markers just prior to surgery, whereas the other four patients had elevated markers but had reached a plateau. The residual mass was successfully resected in all 10 patients. Viable tumor was found in eight patients; the remaining patients had only mature teratoma in the resected specimen. With an average follow-up of 64 months following resection, 5 of 10 patients were alive without evidence of disease. The remaining five patients all had recurrence of disease leading to death within 6 months of resection.
Six patients had more than one site of residual disease. Five patients had rising markers prior to surgery and one had elevated markers that had reached a plateau. The latter patient was the only long-term survivor of salvage surgery. The surgical specimen revealed only necrosis, but serum markers normalized postoperatively. All other patients died of relapsed disease.
Overall, a 37% (6/16) long-term disease-free survival was observed. This study did not confirm the prognostic significance of isolated AFP elevation. However, all five patients with elevated serum markers that had plateaued prior to surgery were long-term survivors. In contrast, only 1 of 11 patients with rising markers prior to surgery was a long-term survivor. A solitary retroperitoneal mass was again associated with a favorable outcome.
These results were confirmed by groups from the UK, Germany, and Japan. Ravi et al reported on 30 marker-positive patients, 17 of whom (57%) were rendered disease-free after salvage surgery.[7] Albers and colleagues achieved similar results in their cohort of 30 chemorefractory patients.[8] Habuchi et al describe 24 chemorefractory patients, 10 (42%) of whom achieved long-term disease-free survival after salvage surgery with or without adjuvant therapy.[9]
In the largest series of patients undergoing salvage surgery, investigators reviewed data from 64 patients with elevated serum markers after second-line chemotherapy. Active germ-cell cancer was detected in 75.8% of these patients. The 5-year overall survival rate was 33.3% with a median survival time for all 64 patients of 15 months (95% confidence interval [CI] = 9-21 months). Postoperative chemotherapy did not improve disease free recurrence in this cohort, as would be expected in patients with largely chemorefractory disease. Four variables were found to be negatively associated with survival: increasing preoperative beta-HCG, elevated AFP (continuous variable), repeat RPLND, and germ-cell cancer in the surgical specimen.[10]
Patients who develop chemorefractory disease represent a particularly challenging, and fortunately relatively small, subset of germ-cell tumor cases. Clinicians are obligated to recognize disease that is unlikely to respond to further chemotherapy and thoroughly evaluate these patients for potentially resectable disease. Cure rates with surgical salvage in this patient population range from 33% to 73%.
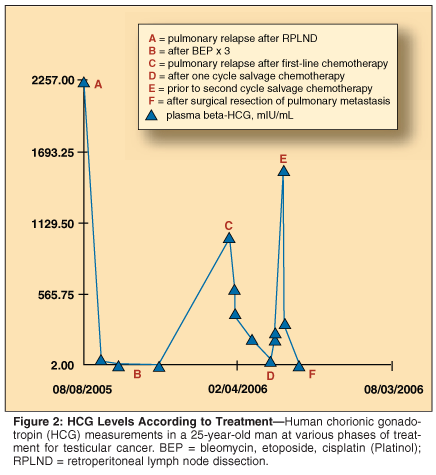
The patient underwent resection of the growing pulmonary nodule. Pathology revealed choriocarcinoma; serum markers normalized postoperatively (see Figure 2). The patient is alive without evidence of disease at 5 months postresection.
Case 2
A 21-year-old man presented with right testicular pain and discomfort referable to a palpable right-sided lower abdominal mass. Right radical orchiectomy revealed a 2-cm nonseminomatous germ-cell tumor comprised of embryonal carcinoma and mature teratoma. Abdominal computed tomography (CT) showed a large multiloculated mass with solid and cystic components in the right retroperitoneum. AFP and HCG were elevated at 1,700 ng/mL and 150 mIU/mL, respectively. Four cycles of BEP chemotherapy were administered, with normalization of serum tumor markers. A repeat CT of the abdomen and pelvis revealed a 5-mm increase in the size of the large retroperitoneal mass (see Figure 3). The patient was also found to have deep-venous thrombosis extending from the iliac vessels into the vena cava.
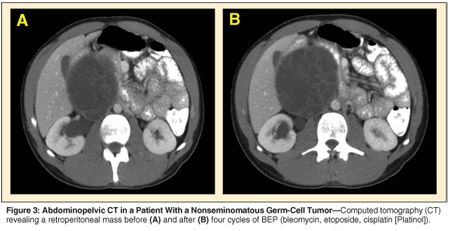
Could this represent anything other than progressive disease?
In 1982, Logothetis et al[11] reported on six patients in whom a growing mature teratoma developed after chemotherapy. Two patients had enlarging retroperitoneal masses and four others had enlarging pulmonary masses. Each was considered to have chemorefractory disease that prompted a surgical salvage approach. Pathology revealed mature teratoma without evidence of malignant histology in all six patients. Four of the specimens consisted of firm masses of cartilage, respiratory epithelium, and smooth muscle, and two others had tense and expansile cysts. No further treatment was given, and all patients remained alive without evidence of disease at a minimum 5-month follow-up.
Three criteria were thus established for the definition of growing teratoma syndrome (GTS): normalization of serum tumor markers, increase in tumor size during or after chemotherapy, and the absence of any nonseminomatous germ-cell tumor component other than mature teratoma at tumor resection. In 2000, Andre et al[12] reported results of treatment and long-term follow-up on 30 male patients with GTS. All patients received primary cisplatin- or carboplatin-based chemotherapy, which resulted in normalization of serum tumor markers. The primary tumor histology was reviewed in 28 patients; 86% (24 of 28) had evidence of mature teratoma.
Complete surgical resection was performed in 24 patients. There was one recurrence of GTS in this group at a median follow-up of 48 months. In the remaining six patients, only partial resection could be performed. Five of these patients developed recurrent GTS at a median follow-up of 54 months. In all but one case, relapse occurred at a previous site of GTS. The 5-year overall survival and progression free survival were 90% and 73%, respectively. The authors concluded that three factors were predictive of the subsequent development of GTS: the presence of mature teratoma in the primary nonseminomatous germ-cell tumor, no reduction in the size of metastases during chemotherapy, and the presence of mature teratoma in postchemotherapy residual masses.
In the large cohort from Indiana University[10] comprising 50 patients with elevated serum tumor markers after first-line chemotherapy who underwent RPLND, residual cancer was found in only 28%. Teratoma was found in 52% and fibrosis in 20%. Clinical parameters predictive of teratoma or fibrosis included first-line chemotherapy only, beta-HCG < 100 ng/mL, and declining serum tumor markers.
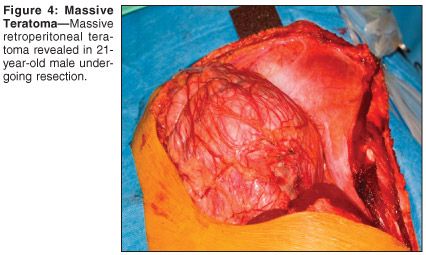
The patient underwent resection of massive retroperitoneal tumor with bilateral retroperitoneal lymph node dissection, vena caval tumor embolectomy, and resection of the right common iliac vein (see Figures 4 and 5). Pathology revealed a 21 × 19 cm, 1,400-g, mature teratoma with cystic differentiation. No residual malignancy was detected in the RPLND specimen. Serum HCG normalized postoperatively. The patient remains without evidence of disease 10 months postoperatively.
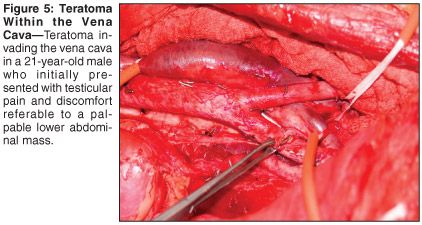
Occasionally, an aggressive surgical approach including vena cava resection, venotomy with thrombectomy, and even aortic replacement are required to achieve disease-free status in patients with bulky abdominal metastatic disease. In a large surgical series from Indiana University[13] comprising 1,180 patients who underwent RPLND, a subgroup of 42 patients required inferior vena cava resection (n = 40) or venotomy with thrombectomy (n = 2). Inverior vena cava thrombus pathology revealed 35% cancer, 45% teratoma, and 20% fibrosis in this series. Pathology of the thrombus reflected the pathology found in the retroperitoneal nodes. The overall survival rate of those with cancer on nodal pathology was 47%, justifying a radical surgical approach in this subset of patients. The complexity of such a procedure mandates that operative candidates with bulky disease be referred to high-volume centers with expertise in urologic oncology.
Case 3
A 31-year-old male was diagnosed with clinical stage I mixed germ-cell tumor of the left testis. Inguinal orchiectomy was performed and pathology revealed embryonal cell carcinoma and teratoma. Serum markers normalized after orchiectomy and he was followed with close surveillance. One year later, he was found to have pulmonary nodules, left-sided retroperitoneal lymphadenopathy, and rising AFP and HCG (52 ng/mL and 180 mIU/mL, respectively). Four cycles of BEP chemotherapy were administered. This resulted in normalization of AFP, but HCG remained elevated at 17 mIU/mL. On xâray, regression was incomplete; positron-emission tomography (PET) did not reveal hypermetabolic activity.
What is the appropriate evaluation of this patient?
Persistently elevated serum tumor markers often indicate the presence of residual disease. However, nonmalignant causes of marker elevation must be ruled out prior to proceeding with further treatment of germ-cell tumors. Upon completion of chemotherapy and surgery, the decrease in serum tumor marker concentrations should follow established half-lives (18 to 36 hours for HCG and 5 to 7 days for AFP).[14]
• Human Chorionic Gonadatropin-HCG, a 45-kDa protein produced by syncytiotrophoblasts, is composed of an alpha subunit shared with luteinizing hormone (LH), follicle-stimulating hormone, and thyroid-stimulating hormone, and a distinct beta subunit. The structural similarity between HCG and LH prevented reliable detection of low levels of HCG until Vaitukaitis et al developed a radioimmunoassay for beta-HCG in 1972.[15] However, there is 80% homology between the beta subunits of HCG and LH. Consequently, falsely elevated levels of HCG are occasionally observed due to cross-reactivity with LH.[16,17]
Orchiectomy and cisplatin-based chemotherapy each cause Leydig cell dysfunction and hypogonadism, which results in compensatory elevation of serum LH.[18] In patients with modest persistent elevation of HCG (< 20 ng/mL), false elevation caused by cross-reactivity with LH can be assessed by administering testosterone and reevaluating the serum marker in 1 to 2 weeks.[19] By reversing the disease- and treatment-induced hypogonadal state, LH will be suppressed and HCG levels should normalize. Testosterone administration will have no effect on HCG produced by residual tumor.
Finally, marijuana use has been implicated as a cause of persistent HCG elevation, and this should be addressed with the patient.[20]
• Alpha-Fetoprotein-AFP is a 70-kDa glycoprotein synthesized in the fetal yolk sac, liver, and intestine. False elevations are associated with malignancies of the gastrointestinal tract (hepatocellular carcinoma and pancreatic, biliary, and gastric cancers) and with liver dysfunction related to hepatitis, cirrhosis, alchoholic liver disease, or biliary tract obstruction.[14]
Testosterone was administered. Two weeks later, the HCG had increased to 20 mIU/mL. Repeat CT of the abdomen and pelvis revealed a 5-mm increase in the size of a residual retroperitoneal mass.
Can a benign histology like teratoma be associated with elevated serum tumor markers?
In rare cases, postchemotherapy cystic teratomas are associated with persistent tumor marker elevation. Van der Gaast and colleagues described two patients with residual marker elevation after primary chemotherapy who were found to have cystic differentiated mature teratoma but no residual malignant disease at RPLND. The cysts contained a high concentration of AFP and HCG. The authors postulated that the persistently elevated tumor markers resulted from leakage of the cyst contents into the plasma compartment.[21]
A larger series from Memorial Sloan-Kettering evaluated the fluid content of 11 patients with postchemotherapy cystic masses who underwent RPLND. All patients had teratomatous elements on primary testicular pathology. Retroperitoneal pathology revealed mature teratoma in nine patients and immature teratoma in the remainder. One patient had foci of malignant transformation. Cystic fluid AFP was elevated in nine patients, two of whom had elevated serum AFP before RPLND that normalized postoperatively. Cystic fluid HCG was evaluated in nine patients and found to be elevated in all of them. Only one patient had elevated serum HCG preoperatively, and this level did not improve after complete resection of the cystic teratoma. The patient subsequently developed pulmonary metastases and died of the disease. Cystic fluid marker concentration appeared to be independent of serum marker level or pathology. This series bolsters the theory that cystic teratomatous masses can result in a slow leak of fluid that may account for persistent serum marker elevation in a rare group of patients.[22]
The patient underwent bilateral nerve-sparing RPLND. Pathology revealed a mature teratoma with cystic differentiation. No residual malignancy was detected in the RPLND specimen. Serum HCG normalized postoperatively. The patient remains without evidence of disease 4 months after RPLND.
GTS is estimated to occur in only 2% to 7% of NSGCT cases,[12] but recognition of this entity is important to prevent patients from receiving inappropriate salvage chemotherapy. Although mature teratoma is considered a benign histology, it can undergo malignant transformation and local progression can result in fatal anatomic complications. These facts, combined with known resistance to primary chemotherapy, makes complete surgical resection the only curative option for mature teratoma.
Summary
Testicular cancer is an uncommon disease associated with very high expectations of successful outcome for nearly all patients. The presence of tumor markers can be a blessing in that these markers often reflect disease status more precisely than traditional methods such as imaging. However, rigorous adherence to the principle that elevated serum markers equate with active malignant disease can lead to therapeutic misadventures. It is always important to interpret the serum markers cautiously, keeping in mind the possibility of false-positive elevation and the association of elevated serum markers with benign teratoma.
Secondly, in this extremely chemotherapy-sensitive disease, it is important and sometimes humbling to realize that aggressive and expert surgery is a critical component in the cure of many germ-cell tumor patients, including those with clear-cut chemotherapy-refractory disease and those with extensive benign teratoma.
The care of these complex young patients demands input from experienced multidisciplinary teams to achieve maximally favorable outcomes.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Bosl GJ, Motzer RJ: Testicular germ-cell cancer. N Engl J Med 337:242-253, 1997.
2. Loehrer PJ, Gonin R, Nichols CR, et al: Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol 16:2500-2504, 1998.
3. Kollmannsberger C, Nichols CR, Bokemeyer C: Recent advances in management of patients with platinum-refractory testicular germ cell tumors. Cancer 106:1217-1226, 2006.
4. Murphy BR, Breeden ES, Donohue JP, et al: Surgical salvage of chemorefractory germ cell tumors. J Clin Oncol 11:324-329, 1993.
5. Wood DP, Herr HW, Motzer RJ, et al: Surgical resection of solitary metastases after chemotherapy in patients with nonseminomatous germ cell tumors and elevated serum tumor markers. Cancer 70:2354-2357, 1992.
6. Eastham JA, Wilson TG, Russell C, et al: Surgical resection in patients with noonseminomatous germ cell tumor who fail to normalize serum tumor markers after chemotherapy. Urology 43:74-80, 1994.
7. Ravi R, Ong J, Oliver RT, et al: Surgery as salvage therapy in chemotherapy-resistant nonseminomatous germ cell tumours. Br J Urol 81:884-888, 1998.
8. Albers P, Ganz A, Hannig E, et al: Salvage surgery of chemorefractory germ cell tumors with elevated tumor markers. J Urol 164:381-384, 2000.
9. Habuchi T, Kamoto T, Hara I, et al: Factors that influence the results of salvage surgery in patients with chemorefractory germ cell carcinomas with elevated tumor markers. Cancer 98:1635-1642, 2003.
10 Beck SD, Foster RS, Bihrie R, et al: Outcome analysis for patients with elevated serum tumor markers at postchemotherapy retroperitoneal lymph node dissection. J Clin Oncol 23:6149-6156, 2005.
11. Logothetis CJ, Samuels ML, Trindade A, et al: The growing teratoma syndrome. Cancer 50:1629-1635, 1982.
12. Andre F, Fizazi K, Culine S, et al: The growing teratoma syndrome: Results of therapy and long-term follow-up of 33 patients. Eur J Cancer 36:1389-1394, 2000.
13. Donohue JP, Thornhill JA, Foster RS, et al: Vascular considerations in postchemotherapy retroperitoneal lymph node dissection. World J Urol 12:182-189, 1994.
14. Morris MJ, Bosl GJ: Recognizing abnormal marker results that do not reflect disease in patients with germ cell tumors. J Urol 163:796-801, 2000.
15. Vaitukaitis JL, Braunstein GD, Ross GT, et al: A radioimmunoassay which specifically measures human chorionic gonadotropin in the presence of human leutinizing hormone. Am J Obstet Gynecol 112:751-758, 1972.
16. Germa JR, Arcusa A, Casamitjana R, et al: False elevations of human chorionic gonadotropin associated to iatrogenic hypogonadism in gonadal germ cell tumors. Cancer 60:2489-2493, 1987.
17. Fowler JE Jr, Platoff GE, Kubrock CA, et al: Commercial radioimmunoassay for beta subunit of human chorionic gonadotropin: Falsely positive determinations due to elevated serum leutinizing hormone. Cancer 49:136-139, 1982.
18. Brennemann W, Stoffel-Wagner B, Helmers A, et al: Gonadal function of patients treated with cisplatin based chemotherapy for germ cell cancer. J Urol 158:844-850, 1997.
19. Catalona WJ, Vaitukaitis JL, Fair WR: Falsely positive specific human chorionic gonadotropin assays in patients with testicular tumors: Conversion to negative with testosterone administration. J Urol 122:126-128, 1979.
20. Saxman SB, Nichols CR, Foster RS, et al: The management of patients with clinical stage I nonseminomatous testicular tumors and persistently elevated serologic markers. J Urol 155:587-589, 1996.
21. van der Gaast A, Hoekstra JW, Croles JJ, et al: Elevated serum tumor markers in patients with testicular cancer after induction chemotherapy due to a reservoir of markers in cystic differentiated mature teratoma. J Urol 145:829-831, 1991.
22. Beck SD, Patel MI, Sheinfeld J, et al: Tumor marker levels in post-chemotherapy cystic masses: Clinical implications for patients with germ cell tumors. J Urol 171:168-171, 2004.