Management of Follicular Lymphoma in the Up-Front and Relapsed Settings
A number of recent treatment advances in the management of follicular lymphoma (FL), including the introduction of the anti-CD20 monoclonal antibody rituximab, have effectively shifted the primary therapeutic goal away from palliation and avoidance of toxicity toward the more proactive objective of extending survival. This paper reviews recent practice patterns in the broad context of the published findings from major phase III randomized trials; it documents potential gaps between trial results and actual practice, and the implications of these for continuing education of oncologists. Forty-three US-based community oncologists participated in a cross-sectional case survey during which 40 documented their management of 186 patients with newly diagnosed FL and 133 patients with relapsed FL, all of whom were treated after January 1, 2008. The findings from this initiative indicate that the majority of these patients did not have any major symptoms at presentation. Additionally, tolerance of and response to treatment, regardless of the regimen employed, were similar across the different age groups studied (<65, 65-74, ≥75 years). Therapies selected by the physicians surveyed in both the up-front and the relapsed settings broadly corresponded to the evidence-based published literature and were supported by treatment guidelines. In addition, a change in the proportional use of bendamustine/rituximab (BR) in the up-front treatment of FL from 2008 to 2010 was observed, suggesting that community oncologists are rapidly incorporating pivotal clinical trial results when deciding on individual patient management strategies.
A number of recent treatment advances in the management of follicular lymphoma (FL), including the introduction of the anti-CD20 monoclonal antibody rituximab, have effectively shifted the primary therapeutic goal away from palliation and avoidance of toxicity toward the more proactive objective of extending survival. This paper reviews recent practice patterns in the broad context of the published findings from major phase III randomized trials; it documents potential gaps between trial results and actual practice, and the implications of these for continuing education of oncologists. Forty-three US-based community oncologists participated in a cross-sectional case survey during which 40 documented their management of 186 patients with newly diagnosed FL and 133 patients with relapsed FL, all of whom were treated after January 1, 2008. The findings from this initiative indicate that the majority of these patients did not have any major symptoms at presentation. Additionally, tolerance of and response to treatment, regardless of the regimen employed, were similar across the different age groups studied (<65, 65-74, ≥75 years). Therapies selected by the physicians surveyed in both the up-front and the relapsed settings broadly corresponded to the evidence-based published literature and were supported by treatment guidelines. In addition, a change in the proportional use of bendamustine/rituximab (BR) in the up-front treatment of FL from 2008 to 2010 was observed, suggesting that community oncologists are rapidly incorporating pivotal clinical trial results when deciding on individual patient management strategies.
TABLE 1
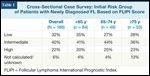
Cross-Sectional Case Survey: Initial Risk Group of Patients with Newly Diagnosed FL Based on FLIPI Score
The fundamental paradigm of initial and long-term management of indolent lymphoma, including follicular lymphoma (FL), changed dramatically with the introduction of the anti-CD20 monoclonal antibody rituximab, used alone or with chemotherapy. This treatment advance effectively shifted the goal of therapy away from palliation and toxicity avoidance toward a more proactive approach of extending survival.[1,2]
With the introduction of alternative and aggressive therapeutic aims, individualized management strategies for FL that incorporate a number of patient- and disease-related characteristics-including tumor burden, histologic grade, and Follicular Lymphoma International Prognostic Index (FLIPI) status, in addition to patient age, performance status, and comorbidities-have become the norm. Thus, the basic clinical algorithm for front-line management (Figure 1) is predicated on two related decisions: Is there a need for systemic treatment? And if so, based on tumor and host factors, what treatments should be considered?
FIGURE 1

Clinical Considerations in the Up-Front Treatment of Follicular Lymphoma
To qualify the impact of these clinical advances on current management of FL, in addition to the 276 cases of MM, the cross-sectional case survey (CCS) included 186 patients with newly diagnosed FL and 133 patients with relapsed FL presenting on or after January 1, 2008. The results are discussed in the context of broadly accepted lymphoma management guidelines and recent findings from major clinical trials.
Follicular Lymphoma: Top-Line Findings from the CCS
In the CCS, approximately two-thirds of patients presented at diagnosis with intermediate or high FLIPI scores, and this distribution did not vary by age (Table 1). In the subset of patients for whom histologic grade was reported, 81% had lower-grade (grade I or II) FL at the time of diagnosis.
TABLE 2

Cross-Sectional Case Survey: Newly Diagnosed Follicular Lymphoma-Symptoms Present at Diagnosis
The majority of patients did not report specific tumor-related symptoms at diagnosis, although a significant minority experienced a more than 10% weight loss or night sweats (Table 2). At the time of initiation of systemic therapy, disease-related symptoms were present in more than 80% of patients (Table 3); this fraction was similar in younger and older patients. These baseline findings are compatible with prior population-based data sets.[2]
Short-Term Outcomes in the CCS
TABLE 3

Cross-Sectional Case Survey: Newly Diagnosed Follicular Lymphoma-Level of Disease-Related Symptoms at the Time the First Treatment Was InitiatedTABLE 4

Cross-Sectional Case Survey: Newly Diagnosed Follicular Lymphoma-Objective Responses and Tolerability with Initial Treatment (by Patient Age)
One important objective of the CCS was to obtain data on global outcomes based on age, regardless of treatment selection, dose, and therapeutic schedule. Of particular interest, the clinical response to treatment and the overall experience with treatment toxicity were similar in all three age groups examined in the CCS (Table 4). Furthermore, of patients 75 years or older, the so-called “very elderly,” almost 80% experienced at least a partial tumor response, and only 1 of 53 patients (2%) had significant or major treatment toxicity.
TABLE 5
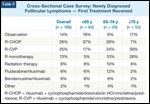
Cross-Sectional Case Survey: Newly Diagnosed Folllicular Lymphoma - First Treatment ReceivedFIGURE 2

Responses to Cross-Sectional Case Survey Question, “Describe the First Treatment This Patient Received for Newly Diagnosed Follicular Lymphoma” (N = 186)
When comparing front-line therapeutic strategies employed in the CCS to the relevant clinical evidence and treatment guidelines, a number of interesting findings emerged. Overall, the most frequently utilized regimens include rituximab alone or in combination with chemotherapy (Table 5 and Figure 2), with patients ≥ 75 years old being more likely to receive R-CVP (rituximab plus cyclophosphamide, vincristine, and prednisone) or rituximab monotherapy than their younger counterparts. In line with the landmark oral presentation at the 2009 American Society of Hematology meeting of the German Study Group of Indolent Lymphomas (StiL) trial data demonstrating greater efficacy and tolerability of front-line bendamustine (Treanda)/rituximab compared with R-CHOP (rituximab plus cyclophosphamide, doxorubicin HCl, vincristine, and prednisone) (Table 6),[3] patients who began front-line treatment in 2010 were significantly more likely to receive this novel regimen (Table 7). Interestingly, this early shift in systemic care occurred without formal FDA approval of bendamustine/rituximab in this setting.
While the practical relevance of a complete clinical response to long-term outcome remains controversial, it is noteworthy that the clinician-assessed complete response rates to front-line therapy for FL in the CCS were somewhat higher than what is typically reported with similar regimens in the published literature (Tables 8 and 9). One possible explanation for this finding may be less rigorous restaging in practice compared with clinical protocols. For example, it has been demonstrated that approximately 60% of patients with FL will present at diagnosis with involved bone marrow, and that induction therapy results in an approximately 40% complete response in the marrow.[4,5] Thus, if postinduction bone marrow biopsies were not performed, one might expect a higher complete response rate. The CCS did not gather data on repeat bone marrow biopsies, but 82% of patients had bone marrow exams at diagnosis.
Short-Term Outcomes for Up-Front Therapy for FL in the CCS and Major Clinical Trials
The following brief and selective review of major outcomes in clinical trials[2,3,5-14] (Table 9) corresponds to the regimens most often utilized in this setting by clinicians in the CCS and allows for an indirect comparison of outcomes reported during this exercise.
Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone
TABLE 6
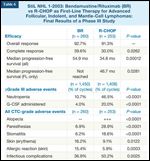
StiL NHL 1-2003: Bendamustine/Rituximab (BR) vs R-CHOP as First-Line Therapy for Advanced Follicular, Indolent, and Mantle-Cell Lymphomas: Final Results of a Phase III Study
As in the recently published LymphoCare registry study of patients who underwent treatment in the United States for FL,[15] R-CHOP was the most common regimen utilized in the CCS (Table 8). Of the 47 patients who received R-CHOP, 77% were considered by their oncologists to have attained complete response. The German Low-Grade Lymphoma Study Group phase III trial of 428 patients with untreated, advanced-stage FL substantiated the superiority of R-CHOP compared with CHOP.[6] In this large and well-conducted clinical trial, R-CHOP resulted in a 96% overall response rate, a 20% complete response rate, and an improvement in overall survival. As alluded to previously, it is probable that the inverse proportions of complete and partial responses noted between the literature and the CCS may be driven by inconsistency in meticulous posttreatment restaging.
In the CCS, only 2 of 47 patients (4%) experienced significant or major complications from treatment with R-CHOP (Table 8). Similarly, in controlled trials, grade 3/4 granulocytopenia occurred after 63% of cycles with R-CHOP but was associated with only a 5% risk of infection. Other adverse events were generally mild to moderate in severity.[6]
Rituximab Plus Cyclophosphamide, Vincristine, and Prednisone
TABLE 7
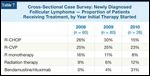
Cross-Sectional Case Survey: Newly Diagnosed Follicular Lymphoma - Proportion of Patients Receiving Treatment, by Year Initial Therapy Started
In almost one-quarter of the cases entered in the CCS, R-CVP was the front-line treatment of choice and resulted in a clinician-reported overall response rate of 98%. In a phase III study of 321 patients with untreated stage III/IV FL, almost 40% of whom had intermediate or high FLIPI scores, the overall response and complete response rates with R-CVP were 81% and 41%, respectively. At a median follow-up of 53 months, the median time to disease progression was 34 months and the median time to treatment failure was 27 months.[4] Fatigue, neutropenia, and back pain were more commonly observed with R-CVP than with CVP. The incidence of grade 3/4 neutropenia with R-CVP was 24%, but no difference was observed in overall infection rates or in the occurrence of neutropenic sepsis.
TABLE 8

Cross-Sectional Case Survey: Newly Diagnosed Follicular Lymphoma - Objective Responses and Tolerability with Top Five Induction Regimens Selected
In the CCS, 42 of 43 patients experienced a partial response or better with R-CVP, and only two (4%) had significant or major complications from treatment (Table 8).
Rituximab Monotherapy
Rituximab monotherapy is an acceptable yet reduced-intensity systemic intervention for newly diagnosed FL,[16] and it was the primary treatment recommended in approximately 12% of CCS cases. In the Swiss Group for Clinical Cancer Research (SAKK) 35/98 phase III study comparing a standard schedule to prolonged rituximab in 202 patients with newly diagnosed or relapsed/refractory FL, the overall response rate was 67% for patients with chemotherapy-naive disease and 46% for patients with pretreated disease.[8,10] At 12 weeks, patients responding to rituximab or with stable disease were randomly assigned to no further treatment or extended rituximab every 2 months for four cycles. The event-free survival was 23 months with prolonged rituximab vs 12 months with the standard schedule and 36 vs 19 months, respectively, for the subset of patients with chemotherapy-naive disease. After a median follow-up of 9.4 years, 35% of all patients and 45% of patients with chemotherapy-naive disease that responded to induction rituximab were still alive and in remission at 8 years.[9] Overall, rituximab was very well tolerated during induction and extended treatment, with low rates of nonhematologic and hematologic toxicity.[8,10]
In the CCS, 19 of 22 patients receiving rituximab monotherapy experienced a partial response or better to treatment, and only 1 patient had significant or major complications.
Bendamustine Plus Rituximab
TABLE 9

Reported Response Rates in Various Trials in Up-Front Treatment of Follicular Lymphoma
The German StiL reported interim findings[17,18] from their phase III non-Hodgkin lymphoma 1-2003 study of 513 patients with untreated indolent and mantle-cell lymphoma who were randomly assigned to bendamustine/rituximab (BR) or R-CHOP, and the initial results were presented at the 2009 American Society of Hematology annual meeting.[3] All patients with FL (N = 279, median age 60) had defined indications for treatment. The overall response rate with BR was 93%, and the complete response rate and progression-free survival were superior to those seen with R-CHOP (Table 6).
Importantly, the tolerability profile was better for BR, with no alopecia and significantly less leukocytopenia, neutropenia, infection, and use of growth factor support. All Common Toxicity Criteria–grade erythema and allergic skin reactions were observed more frequently in patients who received BR (Table 6).[3] Currently, the BR regimen is included as a new front-line therapeutic option by guideline committees.
TABLE 10

Cross-Sectional Case Survey: Relapsed Follicular Lymphoma-Objective Responses and Tolerability With Top Five Selected Most Recent Treatment Regimens
The superior clinical outcomes and favorable tolerability of front-line BR recapitulate prior experiences with this regimen in patients with low-grade non-Hodgkin lymphoma and mantle-cell lymphoma in the first to third relapse, with an overall response rate of 90%, complete response rate of 60%, and median progression-free survival of 24 months.[3]
Consistent with the time frame in which CCS patients were treated, only a limited number received BR induction (n = 11). All cases, however, were reported to have attained an objective response, and no significant or major toxicity was documented by the treating physician (Table 10). Of note, there was evidence of substantial BR uptake following the landmark Rummel presentation, with 31% of those patients who initiated treatment in 2010 receiving BR compared with less than 5% of those who commenced therapy in 2008 or 2009 (Table 7).
Treatment of Relapsed FL
TABLE 11

Cross-Sectional Case Survey: Relapsed Follicular Lymphoma-Level of Disease-Related Symptoms at Initiation of the Most Recent Treatment
Of the 133 cases of relapsed FL entered in the CCS, approximately 50% of patients were reported to be mildly to moderately symptomatic at the initiation of the most recent treatment for relapsed disease, with older and younger patients reportedly experiencing a similar degree of symptomatology (Table 11).
As was seen in the CCS in induction treatment of newly diagnosed FL and multiple myeloma (MM), most patients with relapsed FL experienced at least a partial response to treatment, with the fraction of patients in specific response categories similar in the three studied age groups. Treatment was also generally considered tolerable in all age groups (Table 12).
TABLE 12

Cross-Sectional Case Survey: Relapsed Follicular Lymphoma-Objective Responses and Tolerability with Most Recent Treatment (by Patient Age)
The most commonly utilized treatments were rituximab alone, R-CVP, BR, R-CHOP, and bendamustine monotherapy (Table 10), all of which resulted in objective response rates of > 80%. Again, this is generally higher than response rates reported in the published literature (Table 13).[19-28] As previously described, lack of assessment in the bone marrow may contribute to higher clinician-reported response among these patients relative to published reports. Additionally, it is difficult to compare responses to treatment of relapsed FL in the current era to studies conducted previously, in which patients were exposed to different front-line treatments (Table 13).[19-28] No apparent differences were observed in older vs younger patients in the evaluation of short-term response and tolerability to a single treatment across diverse age groups.
High-dose therapy and stem cell transplant (HDT/SCT) remains a therapeutic option for patients with relapsed FL. Five-year survival rates are 50% to 60%, although treatment-related mortality ranges from 8% to 30%.[27] In the CCS survey, 31 of 133 patients with relapsed FL were considered for HDT/SCT, and 6 actually received this treatment, primarily as later-line therapy.
Discussion
TABLE 13

Select Key Clinical Trials of Approved and Investigational Agents and Radioimmunotherapy in Relapsed Follicular Lymphoma
The findings of the CCS in the FL front-line and relapsed settings mirror what was seen in MM. Specifically, the clinical presentation, tolerance, and response to treatment were similar in all three age groups assessed. This once again suggests that clinicians were able to modify their recommendations of specific treatment regimens for “very old” patients so that therapy yielded favorable short-term outcomes in terms of response and tolerance. It would be worthwhile to further study intricacies of treatment administration in this situation in terms of dosing and schedule to determine how this comparable clinical benefit was achieved.
In addition, the CCS demonstrated that the therapies selected in both the front-line and relapsed FL settings were generally those endorsed by current clinical guidelines and that the specific outcomes in terms of response to and tolerance of these regimens are similar to what has been reported in major clinical trials. The CCS data suggest that the advances in clinical research are being effectively translated in clinical practice and that the various methods of making information accessible to oncologists are allowing the rapid and effective assimilation of clinical research into practice.
References:
References
1. Davis TA, White CA, Grillo-Lopez AJ, et al. Single-agent monoclonal antibody efficacy in bulky non-Hodgkin’s lymphoma: Results of a phase II trial of rituximab. J Clin Oncol. 1999;17(6):1851-7.
2. Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105(4):1417-23.
3. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab is superior in respect of progression free survival and CR rate when compared to CHOP plus rituximab as first-line treatment of patients with advanced follicular, indolent, and mantle cell lymphomas: Final results of a randomized Phase III study of the StiL (Study Group Indolent Lymphomas, Germany). Proc ASH. 2009:Abstract 405.
4. Zhang Q-Y, Foucar K. Bone marrow involvement by Hodgkin and non-Hodgkin lymphomas. Hematol Oncol Clin N Am. 2009;23:873-904.
5. Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579-86.
6. Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725-32.
7. Hainsworth JD, Litchy S, Burris HA 3rd, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20(20):4261-7.
8. Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103(12):4416-23.
9. Ghielmini ME, Schmitz SH, Martinelli G, et al. Long-term follow-up of patients with follicular lymphoma (FL) receiving single agent rituximab at two different schedules in study SAKK 35/98. Proc ASCO. 2009:Abstract 8512.
10. Martinelli G, Hsu Schmitz F.-S., Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol 2010;28:4480-4.
11. Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27(10):1607-14.
12. Czuczman MS, Weaver R, Alkuzweny B, et al. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22(23):4711-6.
13. Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352(5):441-9.
14. Kaminski MS, Estes J, Tuck M, et al. I131-tositumomab monotherapy as frontline treatment for follicular lymphoma: Updated results after a median follow-up of 8 years. Proc ASCO. 2007:Abstract 8033.
15. Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: First report of the National LymphoCare Study. J Clin Oncol. 2009;27(8):1202-8.
16. NCCN. Clinical Practice Guidelines in Oncology-Non-Hodgkins Lymphomas v.1.2010.
17. Rummel MJ, von Gruenhagen U, Niederle N, et al. Bendamustine plus rituximab versus CHOP plus rituximab in the first-line treatment of patients with indolent and mantle cell lymphomas-First interim results of a randomized phase III study of the StiL (Study Group Indolent Lymphomas, Germany). Proc ASH. 2007:Abstract 385.
18. Rummel MJ, von Gruenhagen U, Niederle N, et al. Bendamustine plus rituximab versus CHOP plus rituximab in the first-line-treatment of patients with follicular, indolent and mantle cell lymphomas: Results of a randomized phase III study of the Study Group Indolent Lymphomas (StiL). Proc ASH. 2008:Abstract 2596.
19. Vose JM, Wahl RL, Saleh M, et al. Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2000;18(6):1316-23.
20. Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20(10):2453-63.
21. Witzig TE, Flinn IW, Gordon LI, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20(15):3262-9.
22. Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(27):4473-9.
23. McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825-33.
24. Czuczman MS, Weaver R, Alkuzweny B, et al. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22(23):4711-6.
25. Fowler N, Kahl BS, Rosen P, et al. Bortezomib, bendamustine, and rituximab in patients with relapsed or refractory follicular lymphoma: Encouraging activity in the Phase 2 VERTICAL study. Proc ASH. 2009:Abstract 933.
26. Dutia M, DeRoock I, Chee K, et al. R2: Preliminary results of a phase II study of lenalidomide and rituximab in relapsed/refractory indolent non-Hodgkin’s lymphoma (NHL). Proc ASH 2009:Abstract 1679.
27. Van Besien K, Loberiza FR, Jr, Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102(10):3521-29.
28. Reed-Pease C, Tuscano J. R2: Preliminary results of a Phase II study of lenalidomide and rituximab in relapsed/refractory indolent non-Hodgkin’s lymphoma (NHL). Proc ASH. 2009:Abstract 1679.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.