Management of a Patient With Borderline Resectable Pancreatic Cancer
In this case report, we discuss the presentation, workup, and therapeutic management of a 40-year-old man who presented with borderline resectable, periampullary pancreatic cancer and underwent a margin-negative resection following neoadjuvant chemoradiotherapy.
ABSTRACT: The definition and management of borderline resectability for periampullary pancreatic adenocarcinoma are evolving. In this case report, we discuss the presentation, workup, and therapeutic management of a 40-year-old man who presented with borderline resectable, periampullary pancreatic cancer and underwent a margin-negative resection following neoadjuvant chemoradiotherapy.
The definition of borderline resectability for periampullary pancreatic cancer and the treatment of this condition are evolving. In this installment of Clinical Quandaries, we present the case of a 40-year-old man with the diagnosis of borderline resectable (BR) adenocarcinoma of the pancreas, discuss his treatment course, and review the relevant literature.
Case Reports
Presentation and Workup
A 40-year-old man presented to his primary care physician with a history of abdominal pain for several months and weight loss of 20 lb, which was 9% of his usual adult weight. Shortly before presentation, he experienced jaundice, dark urine, light-colored stools, and generalized pruritus.
The patient’s past medical history was notable only for nephrolithiasis. He was taking no medications and had no known drug allergies. He denied clinically significant alcohol intake or use of either tobacco or illicit drugs. His family history was significant for a paternal uncle with colon cancer and a maternal nephew with leukemia of unknown type. He had no family history of pancreatic cancer. On physical examination, his vital signs were stable, and his weight was 201 lb (20 lb below his baseline). Physical exam revealed significant jaundice, including scleral icterus. On abdominal exam, he had no tenderness or hepatosplenomegaly. His heart rate and rhythm were regular, and his lungs were clear to auscultation. His performance status on the Eastern Cooperative Oncology Group (ECOG) scale was 0.
FIGURE 1
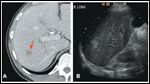
Suspicious Lesion FIGURE 2
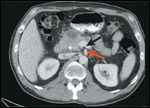
Further CT Findings
Computed tomography (CT) scan of the abdomen and pelvis revealed a complex cystic mass in the head of the pancreas with ill-defined borders and biliary dilatation. A questionable right hepatic lobe lesion was of concern for metastatic disease (Figures 1A and 2). A right upper quadrant ultrasound demonstrated a 3.5 × 1.8 × 2.5 cm cystic mass in the pancreatic head, and a right liver lobe lesion measuring 1.7 cm (Figure 1B).
The initial lab workup revealed a CA 19-9 of 83 U/mL (normal, < 37 U/mL) when he was jaundiced, but this value decreased to 33 U/mL after the jaundice resolved. His total bilirubin (7.2 mg/dL), aspartate aminotransferase (175 U/L), alanine aminotransferase (501 U/L), and alkaline phosphatase (422 U/L) were all markedly elevated at presentation. The remainder of his laboratory tests were within normal limits. Endoscopic retrograde cholangiopancreatography (ERCP) was performed and a biliary stent was placed, resulting in the resolution of the patient’s symptoms and normalization of his lab values. Brushings from the ERCP were nondiagnostic.
The patient then came to our institution for further management. An endoscopic ultrasound (EUS) visualized a 3.5 × 2.2 cm irregular cystic lobulated mass in the pancreatic head, with clear involvement of the portal vein at the confluence with the superior mesenteric vein (SMV), causing limited occlusion (Figure 3). Fine-needle aspiration (FNA) was performed during EUS and revealed well differentiated pancreatic adenocarcinoma with papillary features. The patient experienced a recrudescence of his presenting symptoms and required repeat ERCP to replace an occluded biliary stent.
FIGURE 3
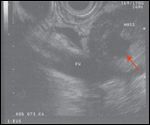
SMV/PV Involvement
The patient was also referred to an interventional radiologist to obtain a pathologic diagnosis of the suspicious liver lesion. Under ultrasound guidance, FNA of the liver lesion was performed. Cytology revealed reactive hepatocytes and fibrosis, consistent with hemangioma (Figure 4).
Treatment
FIGURE 4

Cytologic Findings
The patient was first seen by a general surgeon. Due to the involvement and limited occlusion of the SMV, upfront surgery was not recommended. The patient was referred to medical oncology and radiation oncology for consideration of neoadjuvant treatment.
Weekly gemcitabine (Gemzar) was given initially at a dose of 1,000mg/m2. The patient received four doses of gemcitabine before beginning concurrent chemoradiotherapy with reduced weekly doses of gemcitabine (750 mg/m2). Radiation was targeted to the pancreas and draining regional lymph nodes at 54 Gy in 30 fractions using a four-field, three-dimensional conformal technique. He tolerated radiotherapy well and required no treatment breaks. He experienced grade 2 nausea and vomiting, which resolved with prochlorperazine. The third dose of gemcitabine midway through the radiation course was held due to grade 3 hematologic toxicity (leukopenia), and the subsequent doses were decreased to 375 mg/m2. A repeat CT scan after the completion of neoadjuvant therapy showed shrinkage of the pancreatic head mass and reduced portal vein and SMV involvement. There was no evidence of portal vein occlusion.
Eight weeks after the completion of chemoradiotherapy the patient underwent laparotomy. Intraoperatively, tumor was found to be adherent to the posterior wall of the stomach and to the portal vein at the confluence with the SMV. A classic pancreaticoduodenectomy (Whipple procedure) with partial gastrectomy and partial portal vein resection were performed, including portal vein repair using a saphenous vein patch.
FIGURE 5
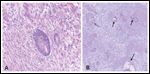
Pathology Specimens From Whipple Procedure After Neoadjuvant Therapy
Pathologic examination of the surgical specimen revealed residual well-differentiated adenocarcinoma, 8 mm in diameter, with surrounding fibrosis. Margins were negative, and three sampled lymph nodes were free of tumor. No venous involvement was noted (Figure 5). Postoperatively, the patient did well. He was placed on continuous venous nutrition, which was discontinued when he was able to tolerate oral intake. He was discharged 10 days after surgery.
Three months after surgery, he restarted chemotherapy, receiving an additional four cycles of gemcitabine (600 mg/m2 with a 3-week-on/1-week-off schedule). He required frequent treatment delays and dose reductions as a result of hematologic toxicity. Since the completion of adjuvant therapy, he has returned to work full-time. His most recent imaging, performed 11 months after surgery, showed no evidence of recurrent disease. His CA 19-9 level, initially elevated at presentation, has remained undetectable.
Discussion
The management of pancreatic cancer is both challenging and controversial. An incidence of 37,600 cases is estimated in the United States for 2008, with 34,290 deaths related to pancreatic cancer.[1] Between 70% and 80% of pancreatic adenocarcinomas present in the periampullary region. The parity of the rates of diagnosis and death for pancreatic cancer highlights both the difficulty in treating this disease and the need for new treatment paradigms. Complete surgical resection remains the sine qua non of curative therapy, but only 20% of patients have resectable disease at presentation.[2] Furthermore, even with negative-margin resection, 5-year survival rates range from 20% to 30%, supporting the role of adjuvant treatment.[2,3] In the following section, we discuss the evidence and rationale supporting adjuvant and neoadjuvant therapy for periampullary pancreatic cancer and the relevance of these data in the management of a case with borderline resectability.
Workup
Over the past decade, technical improvements have enhanced our ability to evaluate and stage pancreatic cancer. EUS is very sensitive for detecting vascular invasion and is especially helpful when the CT findings are equivocal.[4,5] This sensitivity is valuable in determining resectability in certain situations. Additionally, EUS allows for tissue diagnosis via FNA.[6] EUS-directed FNA is preferable to CT-guided FNA because of the much lower risk of peritoneal seeding.[7] EUS is technically demanding, and operator expertise affects both sensitivity and specificity.
CA 19-9 is a sialyated Lewis A blood group antigen commonly expressed in pancreatic cancer; it also can be elevated in several other malignancies and hepatobiliary disease. Estimates of the sensitivity and specificity of CA 19-9 for pancreatic cancer range from 70% to 90%.[8] CA 19-9 can be spuriously elevated by hyperbilirubinemia, so serum levels should be repeated after the resolution of jaundice. CA 19-9 is most useful in monitoring for disease recurrence after surgery since rising levels can be a harbinger of overt relapse.[9]
If biopsy is not feasible for patients with suspected liver metastases, positron-emission tomography (PET) has been shown to be reliable in detecting liver metastases larger than 1 cm.[10]
Adjuvant Therapy
For more than 2 decades, postoperative chemoradiotherapy has been a standard treatment for resectable pancreatic cancer in the United States. This practice was supported by the landmark Gastrointestinal Tumor Study Group (GITSG) trial which showed a dramatic survival advantage for patients receiving chemoradiotherapy with concurrent fluorouracil (5-FU) as compared to patients receiving no further therapy after surgery.[11]
A more recent European Organisation for Research and Treatment of Cancer (EORTC) trial randomized patients in a similar fashion.[12] This trial yielded survival curves that appeared very similar to the GITSG trial, but the difference in overall survival did not reach statistical significance. A recent update confirmed the absence of an advantage for the adjuvant therapy arm, although it appears that patients with cancer of the pancreatic head may have experienced a small benefit. Unfortunately, this trial lacked the statistical power to detect a difference with subset analysis, in part due to the use of a two-sided log-rank test.[13-14]
Subsequently, the Radiation Therapy Oncology Group (RTOG) 97-04 trial showed a strong trend toward a survival benefit with the addition of gemcitabine before and after adjuvant 5-FU–based chemoradiation in patients with resected tumors of the pancreatic head.[15] Current controversy regarding the role of radiotherapy in the adjuvant setting based on European Study Group for Pancreatic Cancer (ESPAC)-1 and Charit Onkologie (CONKO)-001 results is acknowledged, but is not germane to the primary focus of this report.[16-17]
Tumors of Borderline Resectability
Adjuvant therapy is limited by the frequent inability of upfront surgery to achieve an R0 resection. Several single-institution experiences have demonstrated that the presence of positive margins at resection is associated with significantly worse survival, approaching that of patients with inoperable disease.[3,18,19] This is of special importance in patients with BR disease. The definition of BR is controversial, but a working definition includes those tumors at high risk for having microscopic residual disease after surgical resection. To achieve a margin-negative (R0) resection in BR disease, neoadjuvant therapy has been incorporated into some treatment paradigms.
Investigation of the BR subgroup is hindered by lack of concordance of definitions of resectability between institutions. Several organizations, including the National Comprehensive Cancer Network (Table 1), have developed classification systems for pancreatic cancer resectability, but no universal standard has emerged. As a result, the available data are difficult to compare. Recently, Katz et al proposed objective CT-based anatomic criteria to classify BR disease.[20] The adoption of an objective standard definition is vital to the study of this patient subgroup.
Neoadjuvant Therapy
Most series reporting on BR patients have focused on neoadjuvant treatment followed by resection. Histopathologic assessment of tumors after neoadjuvant therapy in pancreatic cancer has consistently demonstrated evidence of therapeutic effect in almost all specimens.[21,22] Katz et al found that 56% of surgery specimens had less than 50% viable tumor cells, while Ammori et al found a high degree of therapy-related fibrosis in two-thirds of patients.[20,23]
As a result of tumor shrinkage, neoadjuvant therapy appears to improve the rates of R0 resections in BR pancreatic cancer.[24] Brown et al reviewed the outcomes of BR patients who underwent neoadjuvant therapy followed by surgery and found an 85% rate of R0 resection.[25] Katz et al found that 41% of their BR patients treated with neoadjuvant therapy ultimately underwent surgery with grossly negative margins, and 94% of these had microscopic negative margins. Median survival was 40 months for patients undergoing resection, compared to 13 months for patients not receiving surgery (P < .001).[20]
Patients with clearly unresectable disease (Table 1) are rarely able to undergo margin-negative surgery even after neoadjuvant therapy, and these patients should be treated with paradigms that do not involve surgery.[26,27]
Surgery
Although the neoadjuvant approach moves surgery to the last step in the treatment sequence, this modality retains its primary importance. In the past, pancreatic surgery was associated with a high mortality, causing some experts to question its utility.[28] Improvements in surgical technique and postoperative care have led to low rates of perioperative mortality with pancreaticoduodenectomy.[29] Optimally, these operations should be performed at higher-volume centers, which have lower operative morbidity and mortality rates.[30] Recent surgical advances include the involvement of vascular surgeons to assist with venous resection, reducing complications and allowing for margin-negative resection even in cases of vessel abutment.[31,32]
Case Management
Based on CT and EUS findings, our patient had a high likelihood of having residual disease after upfront surgery. To address this, we treated him with neoadjuvant therapy consisting of gemcitabine, followed by chemoradiotherapy with concurrent gemcitabine. The tumor shrinkage resulting from neoadjuvant therapy aided the surgeon in obtaining microscopically negative margins. After a recovery period, we administered four cycles of adjuvant gemcitabine.
Summary
The workup and treatment of pancreatic adenocarcinoma is evolving and requires a multidisciplinary team approach. Adjuvant therapy after upfront surgery may be suboptimal for BR tumors due to the risk of an incomplete resection. Early data suggest that neoadjuvant chemoradiotherapy can increase the likelihood of a margin-negative resection for BR tumors. Randomized trials are needed to formally evaluate the efficacy of this approach. The adoption of a standard, objective definition of resectability, based on radiographic findings, would improve the ability to study this patient subgroup.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2008. CA Cancer J Clin 58:71-96, 2008.
2. Li D, Xie K, Wolff R, et al: Pancreatic cancer. Lancet 363:1049-1057, 2004.
3. Yeo CJ, Abrams RA, Grochow LB, et al: Pancreaticoduodenectomy for pancreatic adenocarcinoma: Postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 225:621-633, 1997.
4. Rösch T, Dittler H, Strobel K, et al: Endoscopic ultrasound criteria for vascular invasion in the staging of cancer of the head of the pancreas: A blind reevaluation of videotapes. Gastrointest Endosc 52:469-477, 2000.
5. Snady H, Bruckner H, Siegel J, et al: Endoscopic ultrasonographic criteria of vascular invasion by potentially resectable pancreatic tumors. Gastrointest Endosc 40:326-333, 1994.
6. Raut C, Grau A, Staerkel G, et al: Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg 7:118-126, 2003.
7. Micames C, Jowell PS, White R, et al: Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc 58:690-695, 2003.
8. Goonetilleke KS, Siriwardena AK: Systematic review of carbohydrate antigen (CA 19-9. as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol 33:266-270, 2007.
9. Abrams RA, Grochow LB, Chakraverthy A, et al: Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma: Survival results and observations regarding patterns of failure, radiotherapy dose and CA19-9 levels. Int J Radiat Oncol Biol Phys 44:1039-1046, 1999.
10. Frölich A, Diedrichs CG, Staib L, et al: Detection of liver metastases from pancreatic cancer using FDG PET. J Nucl Med 40:250-255, 1999.
11. Kalser M, Ellenberg S: Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 120:899-903, 1985.
12. Klinkenbijl J, Jeekel J, Sahmoud T, et al: Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230:776-782, 1999.
13. Smeenk, van Eijck CH, Hop WC, et al: Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: Long-term results of EORTC trial 40891. Ann Surg 246:734-740, 2007.
4. Garofalo MC, Regine WF, Tan MT: On statistical reanalysis, the EORTC trial is a positive trial for adjuvant chemoradiation in pancreatic cancer (letter to editor). Ann Surg 244:332-333, 2006.
15. Regine W, Winter K, Abrams R, et al: Fluorouracil vs. gemcitabine chemotherapy and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA 299:1019-1026, 2008.
16. Neoptolemos JP, Stocken DD, Friess H, et al: A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200-1210, 2004.
17. Oettle H, Post S, Neuhaus P, et al: Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 297:267-277, 2007.
18. Sohn T, Yeo C, Cameron J, et al: Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg 4:567-579, 2000.
19. Trede M, Schwall G, Saeger HD: Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 211:447-458, 1990.
20. Katz MH, Pisters PW, Evans DB, et al: Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J Am Coll Surg 206:833-846, 2008.
21. Evans DB, Rich TA, Byrd DR, et al: Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 127:1335-1339, 1992.
22. Talamonti MS, Small W, Mulcahy MF, et al: A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol 13:150-158, 2006.
23. Ammori JB, Colletti LM, Zalupski MM, et al: Surgical resection following radiation therapy with concurrent gemcitabine in patients with previously unresectable adenocarcinoma of the pancreas. J Gastrointest Surg 7:766-772, 2003.
24. Pingpank JF, Hoffman JP, Ross EA, et al: Effect of preoperative chemoradiotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. J Gastrointest Surg 5:121-130, 2001.
25. Brown KM, Siripurapu V, Davidson M, et al: Chemoradiation followed by chemotherapy before resection for borderline pancreatic adenocarcinoma. Am J Surg 195:318-321, 2008.
26. White R, Lee C, Anscher M, et al: Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol 6:38-45, 1999.
27. Kim H, Czischke K, Brennan MF, et al: Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg 6:763-769, 2002.
28. Crile G: The advantages of bypass operations over radical pancreaticoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet 130:1049-1053, 1970.
29. Yeo CJ, Cameron JL, Sohn TA, et al: 650 pancreaticoduodenectomies in the 1990s: Pathology, complications, and outcomes. Ann Surg 226:248-257, 1997.
30. Birkmeyer JD, Warshaw AL, Finlayson SR, et al: Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 126:178-183, 1999.
31. Bold RJ, Charnsangavej C, Cleary KR, et al: Major vascular resection as part of pancreaticoduodenectomy for cancer: Radiologic, intraoperative, and pathologic analysis. J Gastrointest Surg 3:233-243, 1999.
32. Fuhrman GM, Leach SD, Staley CA, et al: Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg. 223:154-162, 1996.