Management of a Patient With Stage IIIA (N2) NSCLC
The appropriate treatment of patients with stage IIIA (N2) non–small-cell lung cancer (NSCLC) is unclear. With this case report and review, we address the history, assessment, and management of a 67-year-old patient with this diagnosis, and then discuss the challenges in managing N2 disease, as well as the roles of systemic therapy, surgery, and postoperative radiation therapy.
ABSTRACT: The appropriate treatment of patients with stage IIIA (N2) nonâsmall-cell lung cancer (NSCLC) is unclear. With this case report and review, we address the history, assessment, and management of a 67-year-old patient with this diagnosis, and then discuss the challenges in managing N2 disease, as well as the roles of systemic therapy, surgery, and postoperative radiation therapy.
The management of patients with stage IIIA (N2) non–small-cell lung cancer (NSCLC) is controversial. In this installment of Clinical Quandaries, we present the case of a man with this diagnosis and review the variety of available treatment options.
Case Report
A 67-year-old male was found to have a mass in the left upper lobe of the lung on a routine chest radiograph that was done as part of preoperative work-up for an elective inguinal hernia repair. Following this, a computed tomography (CT) scan of the chest revealed a 2.0 x 1.4 cm soft-tissue density in the lingula (Figure 1A). An enlarged lymph node (1.4 x 1.1 cm) was also noted in the aortopulmonary (AP) window. A small hypodense lesion was noted in the liver. No other abnormalities were noted on the chest CT scan. The patient subsequently underwent a positron-emission tomography (PET) scan. The lingular soft-tissue density and the AP lymph node were both metabolically active with standardized uptake value (SUV) of 5.0 and 4.8, respectively (Figure 1B). The lesion in the liver was not FDG-avid. There was no evidence of extrathoracic disease.
The patient underwent a CT-guided fine-needle aspiration of the left lingular lesion. The biopsy was consistent with poorly differentiated adenocarcinoma. Immunohistochemical stains were positive for cytokeratin 7 and thyroid transcription factor-1, and negative for cytokeratin 20. These findings were consistent with identifying the lung as the primary site of the cancer. In order to evaluate the liver lesion further, a magnetic resonance imaging (MRI) scan of the liver was obtained. The findings suggested the liver lesion to be a benign cyst.
The patient's past medical history was pertinent for hypertension, peptic ulcer disease, basal cell carcinoma of the skin, and benign prostatic hyperplasia. He was on treatment with atenolol and famotidine for his medical illnesses. The patient did not have known allergy to any medications. He quit smoking cigarettes in 1975 and had a 20 pack-year history prior to that. The patient consumed alcohol on a social basis (two drinks per week). He had no history of exposure to asbestos.
His physical exam was pertinent for a slightly elevated blood pressure of 148/90 mm Hg, reducible right inguinal hernia, and a healed surgical scar in the right upper quadrant of the abdomen from a prior cholecystectomy. His respiratory exam was normal. The patient had an excellent functional status. His performance status on the Eastern Cooperative Oncology Group (ECOG) scale was 0.
Based on the above information, his lung cancer was diagnosed as clinical stage IIIA (T1, N2, Mx). Initial laboratory evaluation revealed normal bone marrow, hepatic, and renal function. Pulmonary function tests revealed a forced expiratory volume in 1 second (FEV1) of 2.6 liters (80% of predicted value).
Treatment
Management of this patient was discussed at a multidisciplinary conference that was attended by thoracic surgeons, medical oncologists, radiation oncologists, and pulmonologists. The patient underwent an outpatient left video-assisted thoracic surgery (VATS) to sample the enlarged AP window lymph node. The biopsy was positive for adenocarcinoma. Based on this, it was decided to administer neoadjuvant chemotherapy. The patient was treated with the combination of cisplatin (75 mg/m2) and docetaxel (Taxotere, 75 mg/m2) every 3 weeks for a total of three cycles. He tolerated the treatment well overall. The adverse events associated with chemotherapy included grade 2 fatigue and anemia, grade 3 neutropenia, and grade 1 nausea.
Following completion of the chemotherapy, the patient underwent a repeat PET/CT scan. The new imaging studies showed a slight reduction in size of the lingular lesion and a near-complete resolution of the AP window lymph node (Figure 2). The latter was non–FDG-avid on the follow-up scan, whereas the primary lesion continued to be PET-positive with a lower SUV of 2.8. His performance status remained excellent.

Subsequently, the patient underwent a left thoracotomy and upper lobectomy with complete mediastinal lymph node dissection. The surgical pathologic evaluation revealed a 2.2 x 1.7 cm adenocarcinoma of the lung along with involvement of the visceral pleura (T2) (Figure 3A). The bronchial and vascular margins were negative. A total of 28 lymph nodes were removed from N1 and N2 stations; only three lymph nodes were positive for adenocarcinoma in the AP window and subcarinal region (Figure 3B). Final pathologic stage was IIIA (T2, N2, M0) adenocarcinoma of the left upper lobe.
The patient recovered from surgery and presented for discussion of further treatment options 4 weeks postoperatively. It was decided that radiation therapy would be administered. The role of chemotherapy in this setting was discussed with the patient. The absence of data to recommend additional chemotherapy was explained, and the patient made an informed decision not to receive any further chemotherapy. He tolerated radiotherapy well and received a total of 50.4 Gy (1 fraction/d x 5.5 weeks).
The patient has been on routine follow-up care, with no evidence of disease recurrence approximately 1 year later.
Discussion
Challenges in Managing N2 Disease
The management of patients with N2 disease is one of the most controversial issues in lung cancer. Approximately 10% to 15% of all lung cancer patients present with stage IIIA disease at the time of diagnosis. Stage IIIA (N2) NSCLC comprises a heterogeneous group of patients, varying from those with solitary microscopic nodal involvement to those with bulky, multistation disease.[1] The 5-year survival rate for patients with pathologic stage IIIA disease is less than 25% with surgical resection alone.[2] Reductions in both systemic and local recurrence are important objectives in the treatment of patients with N2 disease.
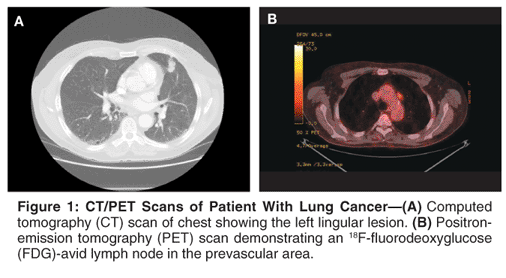
The case described in this article outlines many of the challenges that are intrinsic to the treatment of patients with N2 disease. In the first instance, the enlarged lymph node was not accessible by routine mediastinoscopy and warranted an additional surgical procedure (VATS biopsy) for histologic confirmation. Of note, the PET scan has a false-positive rate of approximately 20% to 30% in staging the mediastinum.[3] On this basis, some would advocate surgical resection with appropriate sampling of the mediastinum as the first step in the treatment of the patient described here.
Our decision to administer systemic therapy before the planned surgery was based on the following facts: (1) Systemic therapy improves survival for patients with early-stage NSCLC[4,5]; (2) upfront surgery is not considered optimal for patients with N2 disease; and (3) administration of neoadjuvant chemotherapy does not increase the risks or the ability to perform a complete resection.[6] The patient received cisplatin and docetaxel, which is a commonly used regimen in the treatment of patients with advanced-stage NSCLC. The utility of this regimen was studied in a phase II study of patients with stage IIIA disease by Betticher et al.[7] In addition to demonstrating the feasibility of this regimen in the preoperative setting, the study noted a promising pathologic complete response rate of approximately 20%.
The patient experienced a good radiographic response to therapy, based on near-complete resolution of the enlarged mediastinal lymph node. The FDG uptake was also lower in the primary tumor. Therefore, he underwent the planned surgical procedure. However, surgical pathology evaluation demonstrated persistent N2 disease. At present, there are no clear guidelines for the management of patients with persistent N2 disease after neoadjuvant therapy and surgical resection. Should these patients continue to receive systemic therapy? If so, should the same chemotherapy regimen as the one used in the preoperative setting be utilized, or something different? Also, what is the role of radiation therapy? The answers to these questions are not well established.
Role of Systemic Therapy
Randomized clinical trials have established a survival benefit with the use of preoperative chemotherapy followed by surgery over that of surgical resection alone in patients with stage III NSCLC.[8,9] Administration of chemotherapy in the preoperative setting does not compromise the ability of patients to undergo the planned surgical procedure. Systemic chemotherapy has also become part of routine care for patients with resected stage II or IIIA NSCLC. Based on these factors, the use of preoperative chemotherapy for patients with N2 disease is a rational approach.
The intervening time between initiation of chemotherapy and the planned surgery allows for identification of patients with rapidly progressive disease, thus avoiding the need for what would have been a futile surgical resection. Preoperative chemotherapy also provides for the ability to treat the micrometastatic disease at an earlier time. Since distant recurrence occurs in a majority of patients with stage III NSCLC, this is thought to be an important factor in their treatment. The lack of evidence to support continued use of the same chemotherapy regimen or selection of a different regimen in this setting was the reason behind our decision not to administer further chemotherapy for this patient.
Role of Surgery in N2 Disease
The role of surgical resection in patients with N2 disease has been addressed by a randomized phase III study reported recently.[10] Patients were randomized to treatment with concurrent chemoradiation followed by surgery or no surgery. There was no difference in overall survival between the two treatment arms, although disease-free survival favored the surgery arm. A higher treatment-related mortality noted in the trimodality arm may have contributed to the lack of survival difference with the addition of surgery. However, for patients who achieved clearance of the positive mediastinal lymph nodes, the 5-year survival rate was 41% with surgical resection following chemoradiation.
Subset analysis from this phase III study demonstrated benefit with surgery in patients who require a lobectomy following induction therapy. Thus, the use of trimodality approach is limited to a small subset of patients with N2 disease. The recent improvements in radiotherapy techniques, chemotherapy regimens, and supportive care have led to the use of concurrent chemoradiation alone as a reasonable treatment option for certain subsets of patients with N2 disease. Whether chemotherapy or concurrent chemoradiation should be the preferred induction regimen remains unanswered. The randomized phase III intergroup study that was initiated to address this issue was closed early due to poor accrual.
Role of Postoperative Radiation Therapy in N2 Disease
Radiation therapy is often used for the treatment of patients with N2 disease. The postoperative radiotherapy (PORT) meta-analysis showed no significant difference in survival for patients with N2 disease with postoperative radiotherapy, although there was a significant trend toward improved survival from the N0 to the N2 subgroups with postoperative radiation therapy. It should be noted that this meta-analysis has been criticized for including unpublished data, patients with inadequate staging, and patients treated with outdated techniques.[11]
An analysis of the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database showed a significant increase in survival for patients with N2 disease who received postoperative radiation therapy. Unfortunately, the SEER database does not indicate which patients received chemotherapy, how the radiation therapy was delivered, or how other confounding factors such as margin status or performance status may have contributed to the recommendation for postoperative radiation therapy.[12] A regression tree analysis concluded that postoperative radiation therapy yielded a significant survival benefit in high-risk and intermediate-risk N2 patients. These subgroups were found to be those patients with at least two positive N2 nodes, or patients with one positive N2 node and T3/4 primary tumors. Of note, 12% of the patients in this study received chemotherapy.[13]
Three randomized trials have addressed the question of postoperative radiation therapy for patients with N2 disease. The Lung Cancer Study Group (LCSG) 773 trial randomized 230 patients with resected stage II or III epidermoid lung cancer to postoperative radiation therapy or observation. Patients with N2 disease had a significant reduction in the overall recurrence rate, but there was no improvement in survival.[14] A Medical Research Council trial randomized 308 patients with resected stage II/IIIA disease to observation or postoperative radiation therapy. Patients with N2 disease had a 1-month improvement in survival, as well as prolonged intervals to local recurrence and the appearance of bone metastasis.[15] The third study randomized 74 patients with resected T1–3, N2 disease to observation or postoperative radiation therapy. There was no significant difference in survival or the rate of locoregional recurrence as initial failure.[16]
Summary
The management of N2 disease requires a multidisciplinary team approach and is often established on a case-by-case basis. Patients with bulky mediastinal adenopathy should be considered candidates for definitive chemoradiation alone, whereas those with microscopic mediastinal nodal involvement may be candidates for neoadjuvant therapy followed by surgery.
Patients with persistent N2 disease detected at the time of surgery should be given postoperative adjuvant chemotherapy, if they had not received prior systemic therapy. It is reasonable to offer postoperative radiation therapy to patients with N2 disease. Improvements in chemoradiation approaches, appropriate risk stratification methods for patients with N2 disease and innovations in noninvasive staging of the mediastinum are all under active investigation and will hopefully lead to improved outcomes for patients with stage IIIA (N2) disease.
Disclosures:
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Andre F, Grunenwald D, Pignon JP, et al: Survival of patients with resected n2 non-small-cell lung cancer: Evidence for a subclassification and implications. J Clin Oncol 18:2981-2989, 2000.
2. Mountain CF, Dresler CM: Regional lymph node classification for lung cancer staging. Chest 111:1718-1723, 1997
3. Bunyaviroch T, Coleman RE: PET evaluation of lung cancer. J Nucl Med 47:451-469, 2006.
4. Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352:2589-2597, 2005.
5. Arriagada R, Bergman B, Dunant A, et al: Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350:351-360, 2004.
6. Pisters KM, Vallieres E, Bunn PA, et al: S9900: A phase III trial of surgery alone or surgery plus preoperative paclitaxel/carboplatin chemotherapy in early stage non-small cell lung cancer: Preliminary results (abstract LBA7012). J Clin Oncol 23(16S):624s, 2005.
7. Betticher DC, Hsu Schmitz SF, Tötsch M, et al: Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 NSCLC: A multicenter phase II trial. J Clin Oncol 21:1752-1759, 2003.
8. Rosell R, Gomez-Codina J, et al: A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 330:153-158, 1994.
9. Roth JA, Fossella F, Komaki R, et al: A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage iiia non-small-cell lung cancer. J Natl Cancer Inst 86:673-680, 1994.
10. Albain K, Swann R, Rusch V, et al: Phase III study of concurrent chemotherapy and radiotherapy (CT/RT) vs CT/RT followed by surgical resection for stage IIIA(pN2) non-small cell lung cancer (NSCLC): Outcomes update of North American Intergroup 0139 (RTOG 9309) (abstract 7014). J Clin Oncol 23(16S):624s, 2005.
11. Port Meta-Analysis Trialists Group: Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet 352:257-263, 1998.
12. Lally BE, Zelterman D, Colasanto JM, et al: Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the Surveillance, Epidemiology, and End Results database. J Clin Oncol 24:2998-3006, 2006.
13. Sawyer TE, Bonner JA, Gould PM, et al: Effectiveness of postoperative irradiation in stage IIIA non-small cell lung cancer according to regression tree analyses of recurrence risks. Ann Thorac Surg 64:1402-1408 (incl discussion), 1997.
14. The Lung Cancer Study Group: Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. N Engl J Med 315:1377-1381, 1986.
15. Stephens RJ, Girling DJ, Bleehen NM, et al: The role of postoperative radiotherapy in non-small-cell lung cancer: A multicentre randomised trial in patients with pathologically staged T1-2, N1-2, M0 disease. Medical Research Council Lung Cancer Working Party. Br J Cancer 74:632-639, 1996.
16. Debevec M, Bitenc M, Vidmar S, et al: Postoperative radiotherapy for radically resected N2 non-small-cell lung cancer (NSCLC): Randomised clinical study 1988-1992. Lung Cancer 14:99-107, 1996.