Management of Patients With Resistant or Refractory Chronic Myelogenous Leukemia
The introduction of imatinib mesylate (Gleevec) has dramatically changed the management and prognostic outlook of patients with chronic myeloid leukemia (CML).
ABSTRACT: The introduction of imatinib mesylate (Gleevec) has dramatically changed the management and prognostic outlook of patients with chronic myeloid leukemia (CML). Despite the outstanding results achieved with imatinib, approximately 20% to 30% of patients may either not respond to therapy or eventually develop resistance or intolerance to the drug. Resistance to imatinib is mediated to a great extent by the emergence of mutations within the tyrosine kinase domain of the BCR-ABL oncogene. A growing number of tyrosine kinase inhibitors (TKIs) with different pharmacokinetic and pharmacodynamic profiles are currently being investigated in clinical trials to determine their efficacy against CML resistant to imatinib therapy. The leading examples of this group of second-generation TKIs are nilotinib (Tasigna) and dasatinib (Sprycel). This review addresses the causes and consequences of imatinib resistance and current management of refractory CML with the second-generation TKIs.
In the absence of effective therapy, patients with chronic-phase chronic myeloid leukemia (CML) invariably progress to a more aggressive phase of the disease termed blastic phase, typically preceded by an accelerated phase of variable duration.[1] The hallmark of the accelerated phase and particularly of the blastic phase is an unregulated overproduction of cells of myeloid origin with an increasing representation of immature forms as the disease progresses. Thus, patients in blastic phase present with a peripheral blood or bone marrow blast percentage of 30% or higher.[1] The estimated risk of transformation to the blastic phase is 5% to 10% per year during the first 2 years after diagnosis and increases to up to 20%–25% per year thereafter.[1,2] Blastic-phase CML is usually resistant to standard chemotherapeutic agents. Prior to the introduction of targeted tyrosine kinase inhibitors (TKIs) for the treatment of CML, the median survival of patients in blastic phase was less than 6 months.
The hallmark genetic abnormality in CML is the balanced translocation t(9;22)(q34;q11.2), which results in the Philadelphia chromosome (Ph). The molecular surrogate of the Ph chromosome is the BCR-ABL hybrid oncogene,[3,4] which encodes for BCR-ABL, a tyrosine kinase with deregulated activity that has been shown to be both necessary and sufficient for the initiation and maintenance of CML.[5]
The addition of imatinib mesylate (Gleevec) to the therapeutic armamentarium for CML has dramatically changed the management and prognostic outlook of patients with this disease. Imatinib is a phenylamino pyrimidine TKI that specifically targets BCR-ABL, KIT, and platelet-derived growth factor receptor (PDGFR) kinases, has proven to be highly active and safe in patients with CML, and has become standard frontline therapy for patients with this disorder. Despite the outstanding results achieved with imatinib, approximately 20% to 30% of patients may either not respond to therapy or eventually develop resistance or intolerance to the drug. The occurrence of BCR-ABL kinase point mutations is the most frequently identified mechanism responsible for resistance to imatinib and other TKIs. In smaller subsets of patients, other mechanisms have been identified as responsible for imatinib resistance.
Clinical Resistance to Imatinib
Definitions
Resistance to imatinib therapy can be segregated into primary (or intrinsic), in which patients exhibit lack of efficacy to TKIs from the onset of therapy, and secondary (or acquired), in which patients experience an initial response to imatinib followed by loss of response after a period of time of variable length. Resistance can be further subdivided into hematologic (lack of normalization of peripheral blood counts), cytogenetic (persistence of Ph chromosome), and molecular resistance (persistence of BCR-ABL transcripts by reverse transcriptase polymerase chain reaction).
TABLE 1

Failure and Suboptimal Response for Untreated Patients in Early Chronic-Phase CML Receiving Imatinib at 400mg Daily
In 2006, on behalf of the European LeukemiaNet, a panel of experts put forth a set of recommendations for the definition of resistance to imatinib that has now become standard. According to this definition, the lack of achievement of a predefined level of response at specific temporal milestones or the loss of hematologic or cytogenetic response at any time defines imatinib failure (Table 1).[6] Patients who meet these definitions of failure to imatinib have an inferior outcome that mirrors that of patients in the pre-imatinib era.[7] Recognizing the importance of the time to achieve the therapeutic goals, this panel also included a group of patients who were defined as having a suboptimal response. These represent patients who cannot be considered to have failed therapy, but whose prognosis has been shown to be less favorable than that of patients with optimal response at the corresponding time points.
In addition, the panel issued a series of therapeutic recommendations for the management of patients who fail imatinib therapy. Although the definitions of imatinib failure provide a useful framework that facilitates the management of patients with CML, the proposals concerning therapeutic intervention have evolved significantly since the time of the issuance of the guidelines, particularly with the emergence of more data regarding the efficacy and safety of second-generation TKIs.
Causes and Consequences
Imatinib mesylate is an orally bioavailable TKI that inhibits the constitutively active BCR-ABL kinase with 50% inhibitory concentration (IC50) values ranging from 0.1 to 0.5 μM.[8-10] In the phase III, randomized International Randomized study of Interferon versus STI571 (IRIS) trial, the efficacy of imatinib was compared to the combination of interferon-alpha and low-dose cytarabine in patients with newly diagnosed CML in chronic phase.[11] After a median follow-up of 60 months, the projected rates of complete hematologic response (CHR) and cytogenetic response (CCyR) were 98% and 87%, respectively.[12] The estimated 5-year survival rate was approximately 90%.
Based on these results, imatinib has become the standard frontline therapy for CML. The initial enthusiasm brought about by the impressive results obtained with imatinib was partially attenuated by the fact that only a small fraction of patients receiving this TKI actually have their disease eradicated at the molecular level.[13] However, nearly two-thirds of patients achieve a major molecular response (3-log reduction in BCR-ABL/ABL transcript levels), which has been associated with 100% survival free from transformation to accelerated or blastic phase when achieved within 12 months.
Still, approximately 20% to 30% of patients will eventually develop resistance to imatinib. In addition, the response rates observed in patients with CML in accelerated or blastic phase were low, and these responses were generally of brief duration.[14] These shortcomings and the development of resistance in 20% to 30% of patients treated in chronic phase have fueled the study of mechanisms of resistance to TKI therapy in CML and the development of novel agents to overcome them.
Resistance to imatinib is mediated to a great extent by the emergence of mutations within the tyrosine kinase domain of the BCR-ABL oncogene. A growing number of distinct BCR-ABL mutations, currently in excess of 60, have been reported, with the frequency of detection among patients with imatinib-resistant CML ranging from 20% to 90%, depending upon the disease phase and the sensitivity of the detection methodology.[15-21] These mutations translate into single amino acid substitutions that tend to cluster on defined functional areas of the BCR-ABL kinase.
BCR-ABL kinase oscillates between an active (open) and an inactive (closed) conformation, depending upon the spatial position of the activation and the P-loops.[22] In the inactive state, the activation loop swings inwardly toward the catalytic site of the enzyme, whereas in the active state, the activation loop flips outwardly to facilitate substrate binding. Another important domain in ABL kinase activation is the P-loop at the amino-terminal end that functions as a docking site for phosphate moieties of ATP. The P-loop region is home to some of the most common mutations found in patients after imatinib failure.[23-25]
Mutations can induce imatinib resistance by distorting the configuration of the BCR-ABL kinase, which results in the inability of the enzyme to adopt the inactive conformation necessary for imatinib binding.[16,22,26,27] Other mutations may affect necessary binding sites for imatinib, thereby preventing adequate attachment of the inhibitors. Different mutations confer different degrees of resistance. The mutation conferring the highest degree of resistance to imatinib and second-generation TKIs such as dasatinib (Sprycel) or nilotinib (Tasigna) is that involving the substitution of the threonine residue at the “gatekeeper” residue 315 for a bulkier isoleucine amino acid. This mutation is frequently identified in patients with imatinib-resistant advanced-phase CML.[28] The threonine residue at position 315 controls access to the active site of the enzyme, thus causing steric clash with imatinib and other TKIs. For that reason, the BCR-ABL T315I mutation represents a formidable challenge for CML clinicians and researchers.
Other mechanisms of resistance have also been identified in CML, including overexpression or amplification of BCR-ABL, clonal evolution, and BCR-ABL–independent mechanisms such as overexpression of the SRC family of kinases (SFKs),[20,29] inhibition of imatinib influx into the cell, or increased efflux. Unlike BCR-ABL kinase mutations, these other mechanisms of resistance are less well characterized.
Managing Imatinib Resistance: Second-Generation TKIs
A growing number of TKIs with different pharmacokinetic and pharmacodynamic profiles are currently being investigated in clinical trials to determine their efficacy against CML resistant to imatinib therapy. These novel TKIs are endowed with enhanced activity against BCR-ABL kinase relative to imatinib and with the ability to target a wider spectrum of protein kinases, some of which play important roles in downstream BCR-ABL–signaling pathways.
The leading examples of this group of second-generation TKIs are nilotinib and dasatinib. Interestingly, the 2-year survival of patients with chronic-phase CML after imatinib failure is better when subsequent therapy consists of nilotinib or dasatinib, compared with those undergoing allogeneic stem cell transplant or other treatment modalities.[7] Other agents such as bosutinib and INNO-406 are also under clinical development for patients with CML after imatinib failure.
Nilotinib
FIGURE 1
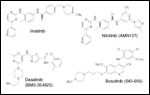
Tyrosine Kinase Inhibitors
-Chemical structures of TKIs currently in clinical development.
Nilotinib (formerly AMN107) is a phenylamino-pyrimidine derivative that was designed based on the crystal structure of imatinib in complex with ABL kinase (Figure 1).[30] Nilotinib has a 20- to 30-fold improved affinity for and inhibitory activity against unmutated BCR-ABL, while preserving activity similar to that of imatinib against KIT (IC50 = 60 nM) and PDGFR (IC50 = 57 nM).[30] A critical advantage of nilotinib over imatinib is its ability to inhibit the tyrosine kinase activity of 32 of 33 BCR-ABL mutants tested (all except for T315I).[23,30] Of note, simultaneous treatment with nilotinib and imatinib resulted in additive/synergistic effects against Ba/F3 cells ectopically expressing multiple BCR-ABL point mutations, but not against T315I.[31] Others like Y253H require relatively high concentrations of nilotinib for full inhibition (ie, high IC50).
Based on this remarkable in vitro activity, nilotinib was first tested in a phase I study in imatinib-resistant CML and Ph-positive acute lymphoblastic leukemia (ALL).[32] A total of 119 patients (17 chronic phase, 56 accelerated phase, 24 myeloid blastic phase, and 22 lymphoid blastic phase/Ph-positive ALL) received nilotinib at doses ranging from 50 to 1,200 mg daily. The maximum tolerated dose was established at 600 mg twice daily. The most frequently encountered grade 3/4 side effects were hematologic, with thrombocytopenia and neutropenia reported in 20% and 13% of patients, respectively. Fluid retention syndromes, weight gain, and rash were frequently seen but were usually mild. Grade 3/4 unconjugated bilirubinemia was reported in 11% of patients receiving 600 mg twice daily and 3% of those receiving 400 mg twice daily, and grade 3/4 elevations of lipase occurred in 5% of patients.[32]
Interestingly, despite a long half-life of approximately 18 hours that would predict a once daily schedule to be optimal, plasma concentrations reached a plateau after daily doses of approximately 600 mg. However, when nilotinib was administered on a twice daily (400 or 600 mg twice daily) schedule, both the plasma concentration and the area under the curve (AUC) were significantly higher than those obtained with the corresponding once daily doses, and this was associated with improved clinical responses.
Based on the activity and safety shown by nilotinib in the phase I trial, a dose of 400 mg twice daily was selected for ongoing phase II studies in patients with CML resistant or intolerant to imatinib therapy (Table 2).[33-35] In the phase II pivotal trial of nilotinib in 321 patients with chronic-phase CML, the CHR rate was 77%. Complete cytogenetic response (CCyR) and partial cytogenetic response (PCyR) rates were 41% and 16%, respectively.[33] Major cytogenetic responses were sustained after 18 months in 84% of patients who attained this response, and the progression-free survival for the total population of patients treated was 64% at 18 months. Nilotinib dosing was escalated to 600 mg twice daily in only 16% of patients. The most frequent grade 3/4 hematologic adverse events included thrombocytopenia (27%), neutropenia (30%), and anemia (9%). Asymptomatic serum lipase elevation was observed in 15% of patients.[33]
The activity of nilotinib was also explored in accelerated-phase (n = 129) and blastic-phase CML (n = 41), with CCyR rates of 19% and 29%–32%, respectively.[34,35] Although nilotinib has the potential for QTc prolongation, in all these studies prolongation of the QTc interval was infrequent, mild, and mostly clinically irrelevant.
Nilotinib is currently approved for the treatment of patients with CML in chronic or accelerated phase who have developed resistance or intolerance to imatinib.
Dasatinib
Dasatinib (formerly BMS-354825) is a potent inhibitor of BCR-ABL (IC50 < 1 nM), SFKs (IC50 = 0.2–1.1 nM), KIT (IC50 = 13 nM), PDGFR-beta (IC50 = 28 nM), and ephrin receptor (EPHA2; IC50 = 17 nM) tyrosine kinases (Figure 1).[36] Unlike imatinib or nilotinib, dasatinib is able to bind both active and inactive conformations of the kinase.[36-38] As a result, in cellular assays, dasatinib inhibited the proliferation of BCR-ABL–positive Ba/F3 and K562 cells with IC50 values of 1.3 and < 1 nM, respectively, and demonstrated high potency against 14 of 15 clinically relevant imatinib-resistant BCR-ABL mutants (except for T315I).[23,37,39]
A phase I study of dasatinib was conducted in 84 patients with CML in all phases (40 chronic phase, 11 accelerated phase, 23 myeloid blastic phase) resistant or intolerant to imatinib or with Ph-positive ALL (n = 10). Patients received dasatinib at daily doses of 15 to 240 mg 5 to 7 days per week in 4-week cycles, either on a once daily or twice daily schedule.[38] A total of 37 patients (92%) in chronic phase carrying a wide variety of BCR-ABL mutations achieved a CHR, including 14 (35%) who also had a CCyR. Grade 3/4 neutropenia or thrombocytopenia was observed in 45% and 35% of patients with chronic-phase CML and in the majority of patients with accelerated phase, blastic phase, or Ph-positive ALL. Fifteen patients had nonmalignant pleural effusions likely related to dasatinib therapy.[38] Based on the short half-life of dasatinib, which resulted in recovery of kinase activity 8 to 12 hours after administration, a twice daily schedule was selected to be studied in phase II studies.
TABLE 2
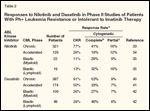
Responses to Nilotinib and Dasatinib in Phase II Studies of Patients With Ph+ Leukemia Resistance or Intolerant to Imatinib Therapy
A series of ongoing phase II studies are addressing the efficacy of dasatinib in patients with CML after imatinib failure (Table 2).[40-42] A total of 387 patients (288 resistant and 99 intolerant of imatinib therapy) with chronic-phase CML have been enrolled in the phase II international multicenter START-C trial (from the SRC/ABL Tyrosine kinase inhibition Activity Research Trials program).[40] The initial dasatinib dose was 70 mg twice daily. The CHR rate was 91%, whereas CCyR or PCyR rates were 53% and 9%, respectively. The average daily dasatinib dose was 101 mg (range = 11–171 mg). Responses were durable, with 84% of patients with imatinib resistance and 97% of those with imatinib intolerance maintaining a major cytogenetic response (McyR) after 24 months. The progression-free survival was 75% for patients with imatinib resistance and 94% for those with imatinib intolerance. Grade 3/4 thrombocytopenia and neutropenia was documented in 48% and 49% of patients, respectively. Nonhematologic toxicity consisted mainly of diarrhea (37%), headache (32%), fatigue (31%), and dyspnea (30%). Pleural effusion was experienced by 27% of patients, being grade 1/2 in 21% and grade 3/4 in 6% of patients. Responses in patients with either accelerated-phase (START-A study)[41] or blastic-phase CML (START-B study)[42] were less satisfactory than those in patients with chronic-phase CML but still with CCyR in 33% of patients treated in accelerated phase, 27% of those treated in myeloid blastic phase, and 46% of patients with lymphoid blastic phase (Table 1).
Before the introduction of second-generation TKIs, the only viable option available for most patients developing resistance to imatinib was dose escalation. A dose escalation from 400 to 800 mg daily resulted in CCyR in nearly 50% of patients with cytogenetic resistance to imatinib with event-free survival after dose escalation of 85%.[43] In an international phase II study, 150 patients with chronic-phase CML resistant to imatinib (400–600 mg/d) were randomized on a 2:1 basis to dasatinib at 70 mg twice daily (n = 101) or imatinib at 800 mg/d (n = 49).[44] Crossover was permitted in the event of progression, lack of MCyR at 3 months, and/or intolerance despite dose reduction (grade 3/4 nonhematologic toxicity). The dose of dasatinib could be escalated to 90 mg twice daily for inadequate response at 3 months.
After a median duration of follow-up of 24 months, CHR rates were 93% and 82% for patients receiving dasatinib and high-dose imatinib, respectively (P = .03). Moreover, dasatinib was also associated with higher MCyR rates 52% vs 33% (P = .02), the main difference being attributable to a higher CCyR rate (40% vs 18%, P = .002).[44] Major molecular responses were also more frequent with dasatinib (29% vs 12%, P = .03). Analyses of progression-free survival (PFS) favored dasatinib (hazard ratio [HR] = 0.14, P < .0001).[44] Unfortunately, this study had a crossover design based on the response at 3 months. Considering that the response after dose escalation occurs after a median of 9 months,[43] early crossover may have precluded a full evaluation of the potential benefit of these two strategies.
In an attempt to improve the safety of dasatinib, different dose schedules were investigated based on a suggestion from the phase I study of decreased frequency of pleural effusions when dasatinib was administered in a single daily dose. Thus, patients with chronic-phase CML who had developed resistance or intolerance to imatinib were randomized to receive dasatinib at a daily dose of either 100 or 140 mg, administered either once daily or in divided doses twice daily. This study showed that a dose of 100 mg once daily was associated with efficacy equivalent to that of 70 mg twice daily (also similar to 50 mg twice daily or 140 mg once daily), but resulted in a significantly lower rate of myelosuppression and pleural effusions. In advanced-phase disease, a similar study testing 70 mg twice daily vs 140 mg once daily showed a similar benefit in toxicity profile with equivalent response rates, but the long-term efficacy has not been established.
Currently, dasatinib is US Food and Drug Administration (FDA)-approved for the treatment of adults with CML in all phases with resistance or intolerance to prior therapy, including imatinib mesylate. For patients in chronic phase, the current standard dose is 100 mg once daily, whereas for patients in accelerated phase or blastic phase it remains 70 mg twice daily.
Bosutinib
Bosutinib (formerly SKI-606) is a potent dual SFK and ABL kinase inhibitor that, unlike imatinib, nilotinib, and dasatinib, has negligible activity against KIT or PDGFR (Figure 1).[45] The clinical activity of bosutinib has been explored in a phase I/II study involving 18 patients with chronic-phase CML resistant or intolerant to imatinib.[46] In the phase I portion of the study, bosutinib was given as a single dose at 400 mg (n = 3), 500 mg (n = 3), or 600 mg (n = 12) daily. Side effects were minimal, the most frequent being mild to moderate diarrhea (87%) and nausea (33%). In contrast to dasatinib,[47] no pleural effusion or pulmonary edema was observed with bosutinib, and these events have been rarely reported with either imatinib or nilotinib,[48] perhaps as a result of the lesser activity of these agents against PDGFR.[45] The dose-limiting toxicity (DLT) of bosutinib occurred at 600 mg daily in the form of severe rash and elevation of transaminases.
A dose of 500 mg daily was selected as the dose for the subsequent phase II portion of the study, which is currently accruing patients in all phases of CML and BCR-ABL–positive B-ALL.[46] Thus far, data are available on 98 patients treated in chronic phase for a median of 3 months.[49] CHR was obtained by 74% of patients resistant to imatinib and MCyR by 41%, including CCyR in 30%.[49] The toxicity profile was remarkably benign, the main grade 3/4 side effects being diarrhea in 6%, rash in 9%, and elevated alanine transaminase (ALT) in 6%.[49] Myelosuyppession was modest, with grade 3/4 thrombocytopenia in 14% and neutropenia in 9%. Among 57 patients with advanced-phase CML (23 accelerated phase, 15 blastic phase, 14 BCR-ABL–positive ALL), 29% in accelerated phase and 25% to 33% in either blastic phase or BCR-ABL–positive ALL had a CHR, with MCyR rates ranging from 50% in accelerated phase to 40% in blastic phase.[50]
INNO-406
INNO-406 (NS-187), another orally bioavailable TKI, inhibits wild-type BCR-ABL with 22 to 55 times the potency of imatinib, as well as ABL-related gene (ARG) and FYN kinases.[29,51] The concentration reached by INNO-406 in the central nervous system, despite being 10% of that detected in plasma, is enough to inhibit the growth of BCR-ABL–positive leukemic cells expressing multiple BCR-ABL mutant isoforms, including F317C/L/V but not T315I.[51]
In a phase I study, 34 patients with CML (21 chronic phase, 7 accelerated phase, 6 blastic phase) and 7 patients with BCR-ABL–positive ALL, all of whom had failed prior imatinib therapy, received INNO-406 after imatinib failure at doses ranging from 30 mg daily to 480 mg twice daily.[52] Prior therapy included two or more TKIs in 32 patients. Common mutations on study entry included Y253H (n = 4), F311L (n = 3), F317L (n = 2), and T315I (n = 2). A total of 27 patients have discontinued INNO-406, 22 of them due to disease progression. Of 7 patients in chronic phase who had failed imatinib (no other prior TKIs), 2 had CCyR, 1 MCyR, and 1 minor cygogenetic response. Among patients who had failed 2 or more TKIs, 1 had a CCyR and 2 had hematologic responses. The dose selected for phase II studies is 240 mg twice daily.
Second-Generation TKIs: A Matter of Time or a Matter of Timing?
Despite the obvious impact of imatinib in the treatment of CML, a subset of patients treated with this TKI will eventually need to switch therapy. In the IRIS study, of the 553 patients who were randomized to imatinib at 400 mg daily, 364 (66%) remain on imatinib and on study after 6 years of follow-up.[53] The main reason for imatinib discontinuation was the lack of a satisfactory therapeutic effect, which occurred in 66 patients (12%).[53]
Although some patients may improve their response with continued imatinib therapy, any given patient not in CCyR faces the competing possibilities of eventually achieving a CCyR vs progressing.[54] It is critical, therefore, to identify at early stages during therapy the subset of patients with a lesser probability of a favorable long-term outcome, in order to institute appropriate therapeutic modifications. Despite the plethora of new TKIs, such modifications have not been fully established and should be considered a work in progress.
The European LeukemiaNet panel of experts recommended an increased dose of imatinib (from 400 mg daily to 600–800 mg daily), allogeneic stem-cell transplantation, or investigational therapies in the event of failure, and stated that these options could be considered in case of suboptimal response to imatinib.[6] Given the lack of evidence at the time these guidelines were issued, one of their major limitations is the absence of specific recommendations as to how, when, or in whom to switch therapy from imatinib to a second-generation TKI. At present, a change in therapy to a second-generation TKI is recommended for patients who meet criteria for imatinib failure. Patients with a suboptimal response need close monitoring, and a dose escalation from 400 to 800 mg is justified. Ongoing studies are investigating the role of a change in therapy to a second-generation TKI in this setting compared to the benefit of dose escalation.
The importance of early intervention has been documented in recent studies. Among patients treated with imatinib dose escalation, the probability of achieving a CCyR is significantly higher if they are treated when they have cytogenetic failure than if the intervention occurs when there is hematologic failure (CCyR rates of 49% vs 12%).
Among patients with chronic-phase CML resistant to imatinib therapy enrolled in the phase II/III dasatinib studies START-C, START-R, and CA180-034,[55] a significantly higher CCyR rate was also reported for patients who were switched to dasatinib upon development of cytogenetic resistance to prior imatinib compared to those who were switched upon hematologic resistance (69% vs 24%). More importantly, these higher rates of CCyR translated into improved progression-free survival, thus underscoring the importance of early intervention with dasatinib in the event of imatinib resistance.[55]
Whether these results will hold up with other TKIs, and whether treatment interventions at the time of suboptimal response change long-term outcomes are issues yet to be clarified.
Conclusions
In recent years, we have made important inroads in the understanding of the mechanisms underlying imatinib resistance, and this progress has been matched with a swift and unrelenting development of alternate therapeutics to manage this problematic subset of patients. Mutations within the ABL kinase domain play a pivotal role in imatinib resistance. Dasatinib and nilotinib, two second-generation TKIs, can overcome the resistance conferred by most ABL mutants and have already been FDA-approved for patients with imatinib-resistant CML, thereby constituting valid and highly effective therapeutic options in this context.
A variety of other agents with the ability to overcome different mechanisms of resistance-bosutinib and INNO-406 are relevant examples-are currently under preclinical and clinical development. However, none of these compounds addresses the highly imatinib-resistant BCR-ABL T315I mutation. Although the data are still preliminary, agents with opportunistic cross-activity against T315I, such as the Aurora kinase inhibitors (MK-0457, XL228, PHA-739358, KW-2449) or homoharringtonine provide a therapeutic alternative for patients with CML carrying this mutation.
Future challenges in the management of patients with imatinib-resistant CML will be the development of TKI combinations with the potential to prevent the emergence of BCR-ABL mutations, and the refinement of therapeutic algorithms to tailor specific TKIs to individual patients according to particular patterns of imatinib resistance.
References:
References
1. Sokal JE, Baccarani M, Russo D, et al: Staging and prognosis in chronic myelogenous leukemia. Semin Hematol 25:49-61, 1988.
2. Sokal JE, Baccarani M, Tura S, et al: Prognostic discrimination among younger patients with chronic granulocytic leukemia: Relevance to bone marrow transplantation. Blood 66:1352-1357, 1985.
3. Bartram CR, de Klein A, Hagemeijer A, et al: Translocation of c-abl oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature 306:277-280, 1983.
4. Groffen J, Stephenson JR, Heisterkamp N, et al: Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell 36:93-99, 1984.
5. Lugo TG, Pendergast AM, Muller AJ, et al: Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 247:1079-1082, 1990.
6. Baccarani M, Saglio G, Goldman J, et al: Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 108:1809-1820, 2006.
7. Kantarjian H, O’Brien S, Talpaz M, et al: Outcome of patients with Philadelphia chromosome-positive chronic myelogenous leukemia post-imatinib mesylate failure. Cancer 109:1556-1560, 2007.
8. Druker BJ, Tamura S, Buchdunger E, et al: Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 2:561-566, 1996.
9. Beran M, Cao X, Estrov Z, et al: Selective inhibition of cell proliferation and BCR-ABL phosphorylation in acute lymphoblastic leukemia cells expressing Mr 190,000 BCR-ABL protein by a tyrosine kinase inhibitor (CGP-57148). Clin Cancer Res 4:1661-1672, 1998.
10. Gambacorti-Passerini C, le Coutre P, Mologni L, et al: Inhibition of the ABL kinase activity blocks the proliferation of BCR/ABL+ leukemic cells and induces apoptosis. Blood Cells Mol Dis 23:380-394, 1997.
11. O’Brien SG, Guilhot F, Larson RA, et al: Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348:994-1004, 2003.
12. Druker B, Guilhot, F, O’Brien S, et al: Long-term benefits of imatinib (IM) for patients newly diagnosed with chronic myelogenous leukemia in chronic phase (CML-CP): The 5-year update from the IRIS study (abstract 6506). J Clin Oncol 24(18S):338s, 2006.
13. Druker BJ, Guilhot F, O’Brien SG, et al: Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355:2408-2417, 2006.
14. Druker BJ, Sawyers CL, Kantarjian H, et al: Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med 344:1038-1042, 2001.
15. Hochhaus A, La Rosee P: Imatinib therapy in chronic myelogenous leukemia: Strategies to avoid and overcome resistance. Leukemia 18:1321-1331, 2004.
16. Shah NP, Nicoll JM, Nagar B, et al: Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2:117-125, 2002.
17. Gorre ME, Mohammed M, Ellwood K, et al: Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293:876-880, 2001.
18. Lowenberg B: Minimal residual disease in chronic myeloid leukemia. N Engl J Med 349:1399-1401, 2003.
19. Corbin AS, La Rosee P, Stoffregen EP, et al: Several Bcr-Abl kinase domain mutants associated with imatinib mesylate resistance remain sensitive to imatinib. Blood 101:4611-4614, 2003.
20. Gambacorti-Passerini CB, Gunby RH, Piazza R, et al: Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias. Lancet Oncol 4:75-85, 2003.
21. Quintas-Cardama A, Gibbons DL, Kantarjian H, et al: Mutational analysis of chronic myeloid leukemia (CML) clones reveals heightened BCR-ABL1 genetic instability and wild-type BCR-ABL1 exhaustion in patients failing sequential imatinib and dasatinib therapy (abstract 1938). Blood 110, 2007.
22. Schindler T, Bornmann W, Pellicena P, et al: Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289:1938-1942, 2000.
23. O’Hare T, Walters DK, Stoffregen EP, et al: In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res 65:4500-4505, 2005.
24. Deininger M, Buchdunger E, Druker BJ: The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 105:2640-2653, 2005.
25. Carter TA, Wodicka LM, Shah NP, et al: Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci U S A 102:11011-11016, 2005.
26. Nagar B, Bornmann WG, Pellicena P, et al: Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res 62:4236-4243, 2002.
27. Hantschel O, Nagar B, Guettler S, et al: A myristoyl/phosphotyrosine switch regulates c-Abl. Cell 112:845-857, 2003.
28. Azam M, Latek RR, Daley GQ: Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell 112:831-843, 2003.
29. Donato NJ, Wu JY, Stapley J, et al: BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood 101:690-698, 2003.
30. Weisberg E, Manley PW, Breitenstein W, et al: Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 7:129-141, 2005.
31. Weisberg E, Catley L, Wright RD, et al: Beneficial effects of combining nilotinib and imatinib in preclinical models of BCR-ABL+ leukemias. Blood 109:2112-2120, 2007.
32. Kantarjian H, Giles F, Wunderle L, et al: Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 354:2542-2551, 2006.
33. Kantarjian H, Hochhaus, A, Cortes, J, et al: Nilotinib is highly active and safe in chronic phase chronic myelogenous leukemia (CML-CP) patients with imatinib-resistance or intolerance (abstract 735). Blood 110, 2007.
34. le Coutre P, Giles FJ, Apperley J, et al: Nilotinib is safe and effective in accelerated phase chronic myelogenous leukemia (CML-AP) patients with imatinib resistance or intolerance (abstract 471). Blood 110, 2007.
35. Giles F, Larson RA, Kantarjian K, et al: Nilotinib in patients (pts) with Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia in blast crisis (CML-BC) who are resistant or intolerant to imatinib (abstract 1025). Blood 110, 2007.
36. Lombardo LJ, Lee FY, Chen P, et al: Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 47:6658-6661, 2004.
37. Shah NP, Tran C, Lee FY, et al: Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305:399-401, 2004.
38. Talpaz M, Shah NP, Kantarjian H, et al: Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 354:2531-2541, 2006.
39. Lee F, Lombardo, L, Camuso, A, et al: BMS-354825 potently inhibits multiple selected oncogenic tyrosine kinases and possesses broad spectrum anti-tumor activities in vitro and in vivo (abstract 675). Proc Am Assoc Cancer Res 46:159, 2005.
40. Stone R, Kantarjian H, Baccarani M, et al: Efficacy of dasatinib in patients with chronic-phase chronic myelogenous leukemia with resistance or intolerance to imatinib: 2-year follow-up data from START-C (CA180-013) (abstract 734). Blood 110, 2007.
41. Guilhot F, Apperley JF, Kim D, et al: Efficacy of dasatinib in patients with accelerated-phase chronic myelogenous leukemia with resistance or intolerance to imatinib: 2-year follow-up data from START-A (CA180-005) (abstract 470). Blood 110, 2007.
42. Gambacorti C, Cortes J, Kim D, et al: Efficacy and safety of dasatinib in patients with chronic myeloid leukemia in blast phase whose disease is resistant or intolerant to imatinib: 2-year follow-up data from the START program (abstract 472). Blood 110, 2007.
43. Jabbour E, Kantarjian H, Atallah E, et al: Impact of imatinib mesylate dose escalation on resistance and sub-optimal responses to standard-dose therapy in patients (pts) with chronic myeloid leukemia (CML) (abstract 1035). Blood 110, 2007.
44. Kantarjian H, Rousselot P, Pasquini R, et al: Dasatinib or high-dose imatinib for patients with chronic-phase chronic myeloid leukemia resistant to standard-dose imatinib: 2-year follow-up data from START-R (CA180-017) (abstract 736). Blood 110, 2007.
45. Puttini M, Coluccia AM, Boschelli F, et al: In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res 66:11314-11322, 2006.
46. Cortes J, Kantarjian, H, Baccarani, M, et al: A phase 1/2 study of SKI-606, a dual inhibitor of Src and Abl kinases, in adult patients with Philadelphia chromosome positive (Ph+) chronic myelogenous leukemia (CML) or acute lymphocytic leukemia (ALL) relapsed, refractory or intolerant of imatinib (abstract 168). Blood 108, 2006.
47. Quintas-Cardama A, Kantarjian H, O’Brien S, et al: Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol 25:3908-3914, 2007.
48. Sawyers CL, Hochhaus A, Feldman E, et al: Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 99:3530-3539, 2002.
49. Cortes J, Bruemmendorf, T, Kantarjian, H, et al: Efficacy and safety of bosutinib (SKI-606) among patients with chronic phase Ph+ chronic myelogenous leukemia (CML) (abstract 733). Blood 110, 2007.
50. Gambacorti-Passerini C, Kantarjian H, Bruemmendorf T, et al: Bosutinib (SKI-606) demonstrates clinical activity and is well tolerated among patients with AP and BP CML and Ph+ ALL (abstract 473). Blood 110, 2007.
51. Yokota A, Kimura S, Masuda S, et al: INNO-406, a novel BCR-ABL/Lyn dual tyrosine kinase inhibitor, suppresses the growth of Ph+ leukemia cells in the central nervous system, and cyclosporine A augments its in vivo activity. Blood 109:306-314, 2007.
52. Kantarjian H, Cortes, J, le Coutre, P, et al: A phase I study of INNO-406 in patients with advanced Philadelphia (Ph+) chromosome-positive leukemias who are resistant or intolerant to imatinib and second generation tyrosine kinase inhibitors (abstract 469). Blood 110, 2007.
53. Hochhaus A, Druker BJ, Larson R, et al: IRIS 6-year follow-up: Sustained survival and declining annual rate of transformation in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib (abstract 25). Blood 110, 2007.
54. Quintas-Cardama A, Kantarjian H, Cortes J: Tyrosine kinase inhibitors for chronic myelogenous leukemia (letter). N Engl J Med 357:1557-1558 (incl author reply), 2007.
55. Kantarjian H, Quintas-Cardama, A, O’Brien, S, et al: Importance of early intervention with dasatinib at cytogenetic rather than hematologic resistance to imatinib (abstract 1036). Blood 110, 2007.