Managing Colorectal Cancer Liver Metastases
The combined-modality care of the patient with colon or rectal cancer metastatic to the liver demands a team approach. It is little wonder that there is much confusion about this topic, given the number of unique treatment options that are delivered in a sequential and reiterative process. The concept of multidisciplinary approaches to complex cancer challenges has been adopted for a variety of tumor types and situations.
ABSTRACT: The treatment of resectable colorectal cancer metastases to the liver has undergone changes with the addition of active chemotherapeutic agents, innovations and definition in the surgical procedures, understanding of the benefits and toxicities of the surgical and chemotherapeutic (cytotoxic and biologic) interventions, and use of the team approach. Patients are initially evaluated for the overall risk of their disease, which includes the standard parameters for disease recurrence and blends in disease and patient comorbidities and likelihood of surgical success. Advanced imaging techniques are mandatory in the initial evaluation. Rather than approaching the patient with sequential, independent therapies and handoff from specialist to specialist, a continuous interaction is required. This article outlines the initial consultation, required team components, surgical decision-making, and use of cytotoxic and biologic agents. The implication is that the best outcomes can only be achieved with the use of all modalities.
The combined-modality care of the patient with colon or rectal cancer metastatic to the liver demands a team approach. It is little wonder that there is much confusion about this topic, given the number of unique treatment options that are delivered in a sequential and reiterative process. The concept of multidisciplinary approaches to complex cancer challenges has been adopted for a variety of tumor types and situations.

Questions of therapy sequencing, therapy-associated morbidity, and disease-related morbidity that limit the use of multiple interventions have necessitated an approach that is data-driven and response-modulated. For patients with colon or rectal cancer metastatic to the liver, both oncologic and functional outcomes are important. The interaction of the modalities must be considered as well as the relative benefits of any single one.
The components of the multidisciplinary team include those with expertise in:
• Diagnostic Radiology-This specialty is important for maintaining consistency during review of the initial films, recommending appropriate additional evaluative studies, and consistently reporting with standard oncologic criteria, such as Response Evaluation Criteria In Solid Tumors (RECIST),[1] on the outcome of therapy. As a subset of the diagnostic radiologist, interventional radiologists may extend the skill set into the invasive procedures.
• Medical Oncology-These team members play a key role in assessing the drug or drug combinations that will have selected tumor responses and patient toxicity. Evaluation of specific toxicities such as those involving the gastrointestinal tract, related to age or comorbidities (hypertension) and the unique interaction of the biologic agents bevacizumab (Avastin) and cetuximab (Erbitux) on wound healing and skin effects.
• Surgical Oncology-The surgical oncologist has a responsibility to assess resectability and properly weigh the surgical complications of bevacizumab associated with wound healing, which are well established. In addition, experience with cetuximab-induced rash and skin changes can be very important in planning a successful, uncomplicated operative procedure.
Surgical Strategies
The overarching question posed to the surgeon is whether all tumors can be destroyed (resected or ablated). In a simpler era, this was a question of whether the tumor could be removed with a negative margin in a single operative procedure. Today, the primary consideration for the surgeon is not so much the location or size of the tumors but rather the volume and function of the liver postoperatively. Mathematical models[2] and the use of computerized tomograms[3] have been developed to estimate the functional liver remnant after surgical resection and correlate that with outcome. Once again, multidisciplinary expertise-including reliance on the radiologist in measuring total liver volume and postoperative liver volume-is critical.
The use of ablative tools such as cryoablation , radiofrequency ablation,[4] or microwave ablation has expanded the population of patients who can be rendered metastasis-free (R0 resection) and in some cases make the need for large resections of otherwise normal tissue unnecessary. The introduction of the two-stage hepatectomy[5] to achieve R0 status is at the same time radical, innovative, and appropriate. Surgeons specially trained in liver resection and hepatobiliary surgery are now accepting the challenge of designing operations and treatment plans that provide maximum resection and extremely low morbidity. The surgical procedures are coupled with other components of care for this patient population. Selection of a surgeon and surgical team with expertise in the assessment of postoperative liver remnant, multimodality surgical techniques, and intraoperative ultrasound is required.
Surgical organizations representing the expertise of surgical oncology and hepatobiliary surgery have come together to define a series of parameters that define resectability.[6] Rather than focusing on the amount of liver removed, they have redirected the resectability analysis on the portion of the liver remaining at the end of the resection. The consensus panel recommended that there be two contiguous Couinaud’s segments with intact vascular inflow and outflow and biliary drainage. Such definitions of resectability often exceed the technical skills of the general surgeon and require specific training in surgical oncology or hepatobiliary surgery. Thus, it is important to recognize that these definitions are made with an expectation of advanced training and experience. Finally, the surgical decision of resectability must also be balanced against patient comorbidities and disease-based risk.
Risk Factors
Several large studies have reviewed risk factors for operative morbidity and the recurrence of colorectal cancer metastatic to the liver.[7-9] The primary disease-specific risk factors include the original stage of the colon or rectal cancer and the disease-free interval between resection of the primary and identification of metastatic disease. Factors related to the metastatic disease include the size of the largest metastasis, number of metastases, patient’s age, CEA level, and ability to completely resect the metastases. These models all exclude patients with extrahepatic disease including metastasis to organs other than the liver, peritoneal implants, and lymph nodes such as those in the porta hepatis and retroperitoneum. The presence of metastatic nodal disease in the porta hepatis has been reported and debated as a relative and not absolute contraindication to curative resection.[10-13]
TABLE 1
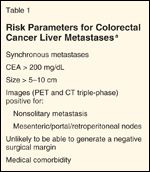
Risk Parameters for Colorectal Cancer Liver Metastases
The above enumerated risk factors are accumulated to determine their overall negative impact on disease-free liver–specific recurrence-free and overall survival. Then, they are used as relative risk factors in modifying the multimodality decision-making (Table 1). For example, the patient with an unfavorable disease-free interval of less than 12 months, multiple tumors, and an age greater than 60 years might be considered for neoadjuvant chemotherapy to assess response of measureable disease, failure outside the liver, and in vivo sensitivity of the metastases to a given agent. These decision-making concepts will be further explored in this paper.
Synchronous Resection of Colorectal Primary and Liver Disease
Although uncommon, the synchronous presentation of resectable liver disease with an intact colon or rectal primary presents a unique scenario. Prior to the widespread use of preoperative chemotherapy and radiation for rectal cancers, the general approach to the primary tumor was resection at initial identification. Currently, with the recognition of lower morbidity and equivalent control with continuous neoadjuvant chemotherapy and concomitant radiation to the rectal tumor and pelvis, the thought process toward initial rectal resection has changed. Similarly, it is more common to consider the synchronous resection of primary colon cancer and liver metastases either at presentation or following neoadjuvant chemotherapy.
Major discussion has centered around the treatment of patients with this presentation, but controversy remains as to whether the operation on the liver and bowel should be performed as a single operative experience or in a staged fashion.[14] Often this decision is based on the intraoperative finding of a liver metastasis when the surgeon is resecting the primary colon tumor. The major issues involved in this situation include the operative approach and skill of the surgeon. For example, it may be difficult to perform a complete, manual assessment of the liver and abdominal cavity intraoperatively due to limited access with the laparoscopic approach. The required skill with intraoperative ultrasound and availability of the correct probe type may also hinder complete evaluation. Finally, intraoperatively there is the inability to obtain fully informed consent for a procedure with known morbidity and mortality. In these situations, it is clearly preferable to complete the diagnostic evaluation postoperatively, seek expert opinion, and stage the patient fully (to include a positron-emission tomography [PET] scan) prior to resection of the liver disease. Preoperative staging with computed tomography [CT] scans that include the liver are more commonly done with larger tumors, and liver disease is often clearly identified and deemed resectable.
If all disease is encompassible at a single operation (based on patient characteristics, tumor characteristics, and surgical skill set-either a single surgeon with skill in resection of bowel tumors and resection of liver metastases or two separate teams), this approach is scientifically sound and preferable. Prospective studies have reviewed the associated morbidity with synchronous or metachronous approaches.[15] The conclusions have clearly favored a combined approach in nearly all clinical scenarios. The total length of stay during a single hospitalization for a synchronous procedure is not thought to exceed that of the two hospitalizations associated with the metachronous approach.
The one legitimate decision point is related to a difficult operative encounter during the resection of the primary disease (ie, colon resection). Such encounters may include excessive blood loss, unprepped bowel, or hypotension. Another cautionary scenario involves a complex or extensive hepatic resection, where morbidity associated with liver failure or infection is of significant concern.
Neoadjuvant Chemotherapy
In most situations, a combined surgical and chemotherapy approach to the metastases is recommended. The timing of treatment with neoadjuvant chemotherapy is crucial, not only for the associated toxicity, but because of the impact of response on the operative intervention. Prior approaches of preoperative chemotherapy for high-risk, resectable metastases that have produced a “best response” led to the ironic conundrum of dealing with lesions that had disappeared. A more confounding situation is one in which a mixed response results in the disappearance of some lesions but persistence of others. One might consider simply following with serial CT and PET scans of the single lesion that has disappeared. However, this is not a reasonable strategy for patients in whom a curative resection could be performed if not for the fact that only a subset of the original multiple lesions requiring resection are visible.
FIGURE 1
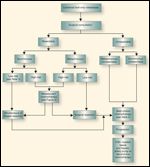
Treatment of Liver-Only Colorectal Cancer Metastases
An interesting retrospective review of patients treated to disappearance of disease demonstrated a remarkable recurrence rate or persistence rate of close to 80%.[16] These data have led to an obligatory sequential evaluation at set time points, usually defined by number of chemotherapy cycles and the status of the lesions. Because the resolution in identification of lesions by CT scan, intraoperative ultrasound, and intraoperative palpation is approximately 1 cm, it is necessary to time the surgery with the change in tumor size. When lesions become smaller than 1 cm they are difficult to identify intraoperatively. In situations where a lesion is not identified, the surgeon should give serious consideration to a blind resection of the area where the tumor formerly resided. In some settings, the goal is reduction of size to change the operative procedure from a lobectomy to segmentectomy or to use ablation instead of resection. In this setting, the effect of therapy is carefully monitored to reach the desired endpoint (Figure 1).
Chemotherapy for Advanced Colorectal Cancer
TABLE 2

Chemotherapy Regimens for Advanced Colorectal Cancer
For decades, the fluorouracil (5-FU)/leucovorin combination was the standard chemotherapy for metastatic colorectal cancer, producing a response rate of 20% to 30% and a median survival time of 12 months. Within the past decade, new agents have led to an improvement in both response rate and median overall survival time (Table 2). Tournigand et al compared FOLFOX (oxaliplatin [Eloxatin], leucovorin, infusional 5 FU) and FOLFIRI (irinotecan, leucovorin, infusional 5-FU) in the first-line setting with protocol-specified crossover to the other regimen as second-line therapy. Both response rates (54%–56%) and median overall survival times (20.6–21.5 months) were similar for the two regimens.[17]
Addition of a biologic agent to chemotherapy-in particular, bevacizumab, an anti–vascular endothelial growth factor (VEGF) monoclonal antibody-was found to be tolerable and improved survival in metastatic colorectal cancer patients. Hurwitz et al compared IFL (irinotecan, bolus 5-FU, leucovorin) with or without bevacizumab in the first-line setting. Patients who received bevacizumab had a longer median overall survival (20.3 vs 15.6 months) and a superior response rate (44.8% vs 34.8%).[18] Following the US Food and Drug Administration (FDA) approval of bevacizumab for use in combination with first-line intravenous 5-FU–based chemotherapy in 2004, many oncologists began combining bevacizumab with FOLFOX or FOLFIRI, which are superior to IFL, without randomized trial evidence. Subsequent randomized trials including the NO16966 and Bolus, Infusional, or Capecitabine with Camptosar-Celecoxib (BICC-C) trials have confirmed the safety and efficacy of bevacizumab in combination with FOLFOX or FOFIRI.[19,20]

Another biologic agent, cetuximab, an anti–epidermal growth factor receptor (EGFR) monoclonal antibody, has also been investigated in randomized trials for metastatic colorectal cancer patients. The phase III CRYSTAL study evaluated the efficacy of adding cetuximab to FOLFIRI in the first-line setting. In this study of 1,198 patients, FOLFIRI plus cetuximab was associated with a longer progression-free survival time (8.9 vs 8 months; P = .048) but no difference in median overall survival.[21] A subgroup analysis of the trial suggests that the clinical benefit of cetuximab is confined to patients whose tumors are KRAS wild-type (approximately 55%–70% of metastatic colorectal cancer patients).
Chemotherapy-Induced Hepatic Damage
Prolonged administration of chemotherapy has been associated with an increased risk of liver damage. Different chemotherapy agents have been associated with different patterns of liver injury. Three main patterns of liver injury can be seen with chemotherapy use: steatosis, sinusoidal injury, and steatohepatitis.
Steatosis is seen in association with 5-FU, oxaliplatin, and irinotecan. Retrospective analysis of the impact of steatosis on outcome suggests that postoperative mortality does not increase, but infectious complications and morbidity do.[22] Sinusoidal injury and other vascular lesions are associated with the use of oxaliplatin. Retrospective analysis shows that morbidity including perioperative bleeding and increased transfusion requirement is increased but not mortality.[23] Steatohepatitis is a more severe form of liver injury than steatosis, characterized by inflammation and ballooning of hepatocytes.
Steatohepatitis is associated with irinotecan and may be associated with an increased 90-day mortality rate due to liver failure postsurgery.[24] However, this finding was not reported in other studies, and some question whether the longer duration of treatment rather than the use of irinotecan is the cause of the increased perioperative mortality rate.
The European Organisation for Research and Treatment of Cancer (EORTC) 40983 study reported data on postoperative morbidity and mortality rates in hepatic resection patients who were randomized to a perioperative FOLFOX4 chemotherapy regimen compared to observation. This phase III study randomized 364 patients with resectable liver metastases to either six cycles of preoperative FOLFOX4 before and six cycles after surgery or surgery alone. This study showed that there was no difference in operative mortality, with both treatment arms having less than a 1% mortality rate. Reversible perioperative complications were more frequent in patients who received chemotherapy than in the observation group (25% vs 16%; P = .04). One patient did not undergo resection because of macroscopic liver damage, which was thought to be most likely related to chemotherapy.[25]
Data on the effects of biologic agents plus chemotherapy are limited with respect to liver damage. Preclinical studies with bevacizumab suggested the possibility of vascular damage and impaired liver regeneration after resection. Several retrospective studies and a postmarketing analysis did not show a major increase in morbidity and mortality, but no randomized prospective study has yet been conducted to provide a definitive answer.[26] Limited safety data exist for anti-EGFR monoclonal antibodies including cetuximab and panitumumab (Vectibix).
Role of Adjuvant Chemotherapy After Hepatic Resection
While the role of adjuvant chemotherapy is well established in stage III colorectal cancer, the role of adjuvant chemotherapy following liver resection is less clear. A recently published meta-analysis evaluated two phase III trials using 5-FU–based chemotherapy regimens. An FFCD (Fdration Francophone de Canclogie Digestive) trial included 173 patients, whereas an ENG (EORTC/National Cancer Institute of Canada Clinical Trials Group/Gruppo Italiano di Valutazione Interventi in Oncologia) trial included 129 patients; the chemotherapy regimen used was bolus 5-FU plus racemic leucovorin ([dl]-leucovorin) or levoleucovorin ([l]-leucovorin). In this pooled analysis of 278 patients, median progression-free survival was 27.9 months in the chemotherapy arm compared with 18.8 months in the observation arm, which did not reach a statistical significance (P = .058). Median overall survival was 62.2 months in the chemotherapy arm compared with 47.3 months in the observation arm (P = .095). Adjuvant chemotherapy was independently associated with both progression-free survival and overall survival in multivariable analysis. The authors concluded that there was a marginal statistical significance in favor of adjuvant chemotherapy with a bolus 5-FU–based regimen in patients with completely resected liver metastases.[27]
Role of Hepatic Artery Infusion Therapy After Hepatic Resection

Hepatic artery infusion chemotherapy maximizes drug exposure to liver metastases by delivering the drug directly via the hepatic artery and utilizing drugs such as floxuridine, which has an extraction rate of over 94% in the liver during the first pass. Some clinical evidence suggests a benefit for the use of hepatic artery infusion in patients with resected liver metastases.
A single-institution study randomized 156 patients into postoperative hepatic artery infusion with floxuridine plus systemic 5-FU with or without leucovorin vs systemic therapy alone. An updated analysis with a 10-year median follow-up reports a significantly greater progression-free survival rate (31.3 vs 17.2 months; P = .02) and a trend toward improved overall survival (68.8 vs 58.8 months; P = .10) in the combined-therapy group compared to the monotherapy group.[28] An intergroup study randomized 109 patients into postoperative hepatic artery infusion with floxuridine plus infusional systemic 5-FU vs observation alone. This trial showed a significantly longer 4-year recurrence-free survival rate (46% vs 25%; P = .04) and a 4-year hepatic recurrence–free survival rate (67% vs 43%; P = .03) favoring the adjuvant therapy group. The median survival time, however, was not significantly different (34 vs 47 months; P = .19).[29]
Widespread implementation of this technique has been limited by the expertise required for optimal pump placement and the management of hepatobiliary toxicity of floxuridine hepatic artery infusion. Also, there is no clear evidence that hepatic artery infusion chemotherapy alone improves overall survival, despite control of liver metastases, due to the development of life-limiting extrahepatic metastases. Furthermore, in the era of modern systemic chemotherapy regimens that produce a high response rate and improved survival time, the role of hepatic artery infusion is unclear. Unfortunately, a phase III trial (National Surgical Adjuvant Breast and Bowel Project [NSABP] C-09) of oxaliplatin plus capecitabine (Xeloda) with or without hepatic artery infusion of floxuridine in patients with resected or ablated liver metastases failed to accrue sufficient patients and was prematurely closed. Given the uncertainty of its benefit in the modern systemic chemotherapy era and the expertise required, the current use of this modality should be restricted to centers experienced with the technique.
Role of Perioperative Chemotherapy for Resectable Liver Metastases
FIGURE 2
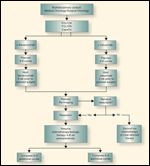
Neoadjuvant Chemotherapy Strategy for Colorectal Cancer Metastases
Potential advantages of administering chemotherapy prior to surgery exist even in the setting of resectable liver metastases. Neodjuvant chemotherapy can provide in vivo chemoresponsiveness information, avoid potential delay of addressing microscopic extrahepatic disease (and may thus improve the cure rate), increase the rate of complete resection (R0), facilitate limited hepatectomies, and identify aggressive disease (thus avoiding ineffective therapy).
Potential disadvantages of neoadjuvant therapy must also be considered. One main concern is that chemotherapy can cause liver damage, resulting in increased morbidity and mortality. The other concern is that response to chemotherapy may result in the disappearance of one or more liver metastases. Studies show that radiologic response often does not correlate with complete pathologic response, and thus a high potential for cancer recurrence. Although data suggest that progressive disease while on neoadjuvant chemotherapy portends a poor prognosis, surgery remains an important treatment modality that can produce long-term survival.
Clinical Evidence
Data from the EORTC 40983 study provide support for perioperative chemotherapy. This phase III trial randomized patients with up to four resectable liver metastases into six cycles of FOLFOX4 before and after surgery or surgery alone. The study showed an absolute increase of 8.1% in the 3-year progression-free survival rate (HR = .77; P = .041) in the perioperative chemotherapy group. Preoperative chemotherapy was relatively well tolerated, and the response rate was 43% with 3% achieving a complete response. The total lesion diameter was reduced by about 25% after chemotherapy. A similar number of patients in both groups received potentially curative resection. Only 7% of patients progressed during chemotherapy, and few of these patients were able to undergo subsequent surgical resection.
Case Report: A Woman With Fatigue, Colon Cancer, and Liver Nodules
The patient is an active 40-year-old woman with a 4-week history of fatigue and severe iron-deficiency anemia (hemoglobin = 8 g/dL). She received 2 units of packed red blood cells, which resolved her fatigue. She was otherwise asymptomatic. Colonoscopy identified a circumferential mass involving the ascending colon. The biopsy reported a poorly differentiated invasive adenocarcinoma. Serum carcinoembryonic antigen (CEA) was elevated at 9.4 ng/mL. Preoperative triple-phase computed tomography (CT) scan of the liver and positron-emission tomography (PET)/CT fusion scan showed a hypermetabolic circumferential lesion involving the ascending colon close to the cecum, measuring 4.6 × 3.8 cm. In addition, two liver nodules were noted. The first, located in segment 8 of the right lobe of the liver, measured 2.9 × 2.2 cm. The second was located near the medial aspect of the right lobe of the liver at the junction of segment 7 and segment 1 and measured 1.3 × 1.5 cm.
The patient was started on the modified FOLFOX6 chemotherapy regimen (oxaliplatin, infusional fluorouracil, and leucovorin) every 2 weeks. She received three cycles of modified FOLFOX6. Serum CEA declined to 2.2 ng/mL, and CT scan showed minor tumor shrinkage with no new liver lesions or extrahepatic disease. She underwent a right hemicolectomy, resection of the segment 8 metastasis with biopsy, and radiofrequency ablation of the segment 7/1 lesion. The pathology report confirmed the presence of two liver metastatic nodules, with a clear surgical margin on the segment 8 lesion.
Five weeks postoperatively, chemotherapy was resumed. Bevacizumab was added to a modified FOLFOX6 regimen 7 weeks postoperatively. Six weeks following the completion of the chemotherapy (total of 12 cycles), the CT scan showed no evidence of disease recurrence, and serum CEA was 0.0 ng/mL.
As previously discussed, no increased postoperative mortality was seen in the perioperative chemotherapy group; both groups had a 1% mortality rate. Reversible postoperative complications, including bleeding, biliary fistula, hepatic insufficiency, and intra-abdominal infection, were increased in the perioperative chemotherapy group (25% vs 16%; P = .04). One patient did not undergo hepatic resection due to macroscopic liver damage, thought to be most probably due to chemotherapy effects.[25]
This study showed that a short duration of preoperative chemotherapy improves progression-free survival with increased but manageable complication rates. While few patients showed disease progression during chemotherapy, this may be regarded as a biologic marker for poor prognosis, and salvage chemotherapy prior to surgery may be an appropriate option. This study, however, does not compare preoperative vs postoperative chemotherapy. The ideal duration of chemotherapy, both preoperative and postoperative, is not clear, but based on the EORTC trial, a total of 6 months including pre- and postsurgery may be considered reasonable. It is important to consider that, due to the risk of liver damage and the risk of radiographic complete response, the duration of preoperative chemotherapy should be kept short so that the complete resection of liver metastases can be achieved. Moreover, it is not clear which chemotherapy regimen is optimal.
As previously discussed, there is no major difference between the FOLFOX and FOLFIRI regimens in terms of response rate, and therefore it would be reasonable to use either regimen. Whether biologic agents should be added to chemotherapy is less certain. The addition of bevacizumab to chemotherapy increases the response rate and prolongs median overall survival time in metastatic colorectal cancer patients. Several groups reported the safety and feasibility of administering bevacizumab with chemotherapy prior to hepatic resection. However, no prospective randomized study is available to help clarify this issue.
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Bevacizumab (Avastin)
Capecitabine (Xeloda)
Cetuximab (Erbitux)
Floxuridine
Fluorouracil (5-FU)
Irinotecan
Leucovorin
Oxaliplatin (Eloxatin)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Similarly, the role of preoperative cetuximab on wild-type KRAS patients with resectable liver metastases is unclear. In a subset analysis, Van Cutsem et al reported that more patients with initially unresectable metastatic disease were able to undergo complete surgical resection with the addition of cetuximab to FOLFIRI (4.8% vs 1.7%; P = .002; Figure 2).[21]
Conclusion
Advances in chemotherapy regimens, targeted biologic agents, surgical techniques, and radiation techniques necessitate the importance of well-coordinated multidisciplinary care to achieve optimal outcomes in colorectal cancer patients with liver metastases: A radiologist, medical oncologist, and hepatobiliary surgeon should be closely involved in deciding the best course of therapy and timing of surgery. Following complete surgical resection and postoperative chemotherapy, close follow-up with imaging studies should be performed.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Jaffe CC: Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol 24:3245-325, 2006.
2. Vauthey JN, Abdalla E, Doherty D, et al: Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 8:233-240, 2002.
3. Capussotti L, Muratore A, Baracchi F, et al: Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg 143:978-982, 2008.
4. Feliberti E, Wagman LD: Radiofrequency ablation of liver metastases from colorectal carcinoma. Cancer Control 13:48-51, 2006.
5. Adam R, Delvart V, Pascal G, et al: Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg 240:644-658, 2004.
6. Charnsangavej C, Clary B, Fong Y, et al: Selection of patients for resection of hepatic colorectal metastases: Expert consensus statement. Ann Surg Oncol 13:1261-1268, 2006.
7. Mann C, Metcalfe M, Leopardi L, et al: The clinical risk score: Emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg 139:1168-1172, 2004.
8. Yasui K, Hirai T, Kato T, et al: A new macroscopic classification predicts prognosis for patient with liver metastases from colorectal cancer. Ann Surg 226:582-586, 1997.
9. Kattan M, Gönen M, Jarnagin W, et al: A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg 247:282-287, 2008.
10. Laurent C, Sa Cunha A, Rullier E, et al: Impact of microscopic hepatic lymph node involvement on survival after resection of colorectal liver metastasis. J Am Coll Surg 198:884-891, 2004.
11. Jaeck D, Nakano H, Bachellier P, et al: Significance of hepatic pedicle lymph node involvement in patients with colorectal liver metastases: A prospective study. Ann Surg Oncol 9:430-438, 2002.
12. Adam R, de Haas R, Wicherts D, et al: Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol 26:3672-3680, 2008.
13. Wagman LD: Expanded criteria for surgery for liver metastases: Thoughtful science or diamond mining? J Clin Oncol 26:3663-3664, 2008.
14. Minagawa M, Yamamoto J, Miwa S, et al: Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg 141:1006-1012, 2006.
15. Martin R, Paty P, Fong Y, et al: Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg 197:233-241, 2003.
16. Benoist S, Brouquet A, Penna C, et al: Complete response of colorectal liver metastases after chemotherapy: Does it mean cure? J Clin Oncol 24:3939-3945, 2006.
17. Tournigand C, André T, Achille E, et al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 22:229-237, 2004.
18. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004.
19. Saltz LB, Clarke S, DÃaz-Rubio E, et al: Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 26:2013-2019, 2008.
20. Fuchs CS, Marshall J, Mitchell E, et al: Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from BICC-C study. J Clin Oncol 25:4779-4786, 2007.
21. Van Cutsem E, Köhne CH, Hitre E, et al: Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408-1417, 2009.
22. Kooby DA, Fong Y, Suriawinata A, et al: Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg 7:1034-1044, 2003.
23. Aloia T, Sebagh M, Plasse M, et al: Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol 24:4983-4990, 2006.
24. Vauthey JN, Pawlik TM, Ribero D, et al: Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 24:2065-2072, 2006.
25. Nordlinger B, Sorbye H, Glimelius B, et al: Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomized controlled trial. Lancet 371:1007-1016, 2008.
26. Bilchik A, Hecht JR: Perioperative risks of bevacizumab and other biologic agents for hepatectomy: Theoretical or evidence based? J Clin Oncol 26:1786-1788, 2008.
27. Mitry E, Fields A, Bleiberg H, et al: Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: A pooled analysis of two randomized trials. J Clin Oncol 26:4906-4911, 2008.
28. Kemeny NE, Gonen M: Hepatic artery infusion after liver resection. N Engl J Med 352:734-735, 2005.
29. Kemeny MM, Adak S, Gray B, et al: Combined modality treatment for resectable metastatic colorectal carcinoma to the liver: Surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy-an intergroup study. J Clin Oncol 20:1499-1505, 2002.