A Multidisciplinary Approach to Neoadjuvant Therapy for Primary Operable Breast Cancer
While the administration of neoadjuvant chemotherapy is recommended for women with locally advanced breast cancer, its use in the primary operable setting has been debated and is the focus of this review.
Neoadjuvant therapy may provide advantages to some women with primary operable breast cancer. Compared to the administration of the same regimen in the adjuvant setting, neoadjuvant chemotherapy does not improve survival outcomes, but may provide other benefits. Neoadjuvant therapy is associated with improved rates of breast-conserving therapy, may offer prognostic information, and enables assessment of in vivo response to therapy. Women who achieve a pathologic complete response following neoadjuvant therapy are expected to have a superior outcome compared to those with extensive residual disease. The neoadjuvant setting has been an attractive area of research for identifying new effective treatment strategies while minimizing treatment-related adverse events, studying drug mechanisms of action, and developing clinically applicable prognostic and predictive biomarkers in an attempt to individualize therapy. In the primary operable setting, it is of great importance to define treatment goals, to select proper candidates for the approach, to assess baseline tumor characteristics, and to provide optimal multidisciplinary monitoring during and following the neoadjuvant therapy.
The improvements in long-term breast cancer–related outcomes in recent decades, including disease-free and overall survival, are attributed to both early screening and optimal multidisciplinary therapy. Traditionally, women undergo a definitive surgical procedure-generally a mastectomy or a lumpectomy with axillary lymph node assessment-to allow for accurate pathologic staging. Because adjuvant systemic therapy significantly improves long-term breast cancer–related outcomes, almost every woman with early-stage disease will be recommended systemic therapy, which may include chemotherapy, endocrine manipulations, targeted therapies, or a combination of these treatment approaches.[1] Systemic treatment recommendations are made based on estimates of risk of recurrence, age, and competing morbidities.
Neoadjuvant therapy, also designated primary systemic or preoperative therapy, was initially employed to downstage inoperable tumors to allow for definitive surgery. Once the long-term benefits of adjuvant therapy were recognized, it was postulated that administration of chemotherapy prior to surgery might be more effective at eradicating micrometastatic disease and therefore could result in improvements in long-term outcomes. Two decades of investigation have demonstrated equivalent survival benefits for the administration of chemotherapy before or after surgery. The neoadjuvant approach may allow for other benefits such as enhancement of breast conservation, but may also be associated with several limitations. It is therefore of great importance to define treatment goals, to select proper candidates, to assess baseline tumor characteristics, and to provide optimal multidisciplinary monitoring during and following neoadjuvant therapy.
Aims of Neoadjuvant Therapy
The primary aims of neoadjuvant therapy for breast cancer are to improve surgical outcomes, to assess response to therapy, and to achieve long-term survival.[2] In patients with locally advanced breast cancer, response to neoadjuvant therapy may lead to improved overall outcomes by allowing for definitive local therapy. In patients with primary operable disease, the neoadjuvant approach provides equivalent disease-free and overall survival compared to those treated with adjuvant therapy, but is associated with improved rates of breast-conserving therapy (BCT).[3] While the administration of neoadjuvant chemotherapy is recommended for women with locally advanced breast cancer, its use in the primary operable setting has been debated and is the focus of this review.
In addition to improvement of surgical outcomes, neoadjuvant therapy may provide other advantages. Response to treatment in an individual woman may predict her long-term outcome. Despite the varied definitions of pathologic complete response (pCR) in trials completed to date, it has been consistently demonstrated that pCR is associated with improved disease-free and overall survival.[4] Women without residual invasive and noninvasive tumor cells in the breast and in the axillary nodes have substantially improved outcomes compared to women with similar stage and tumor characteristics and extensive residual disease. Furthermore, clinical response to therapy can be assessed as early as following one to two cycles and may help predict who will achieve a pCR. Those who appear to be resistant to the chosen treatment should be considered for an alternative therapy, an option that is not currently possible when adjuvant therapy is administered. Therefore, observation of response to treatment can be used as a surrogate marker for disease outcome and treatment may be altered to enhance response.
In addition, because of easy access to the tumor and the ability to assess response to treatment, neoadjuvant therapy has commonly been employed in clinical trials. While administering standard and novel agents, researchers can rapidly investigate the efficacy of the treatment, study drug mechanism of action, and examine potential predictive biomarkers of response to specific treatments. Established or novel imaging techniques may also be utilized to predict response to therapy and long-term outcome using relatively small numbers of patients.
Selection of Patients
A careful staging evaluation should be performed prior to the decision to administer neoadjuvant therapy. Knowledge of the tumor stage and histologic features can help predict who will likely respond to a particular treatment. An International Expert Panel, convened three times over the past decade, has recommended that clinicians should consider neoadjuvant chemotherapy in any patient with primary operable disease for whom adjuvant chemotherapy is clearly indicated.[2] Patient and tumor characteristics can help predict those who will respond to, and therefore benefit from, chemotherapy.
Tumor characteristics that predict an improved response to neoadjuvant chemotherapy include absent or low expression of estrogen receptor (ER), high Ki67 or another proliferation index, high grade, and ductal pathology.[5] Improved response to neoadjuvant chemotherapy and high pCR rates are more likely in women with tumors that amplify or overexpress HER2/neu (HER2-positive) or tumors that lack ER, progesterone-receptor (PR), and HER2 (so-called “triple-negative” breast cancer) compared to women with tumors that are low grade or those that express ER. Women with HER2-positive tumors who are candidates for neoadjuvant chemotherapy should receive a trastuzumab (Herceptin)-based regimen.[6]
While women who do not obtain a pCR have a worse outcome compared to those who achieve pCR, women with hormone receptor–positive tumors are expected to have overall improved outcomes compared to those whose tumors lack ER, likely due to prolonged adjuvant hormone therapy use in the hormone receptor–positive women.[7] For example, women with lobular carcinoma do not respond well to neoadjuvant chemotherapy but have improved survival outcomes.[8] Indeed, recent recommendations suggest that women with large lobular tumors are not good candidates for neoadjuvant chemotherapy, as responses are few and chance for posttreatment breast conservation is low. Together, these observations suggest that treatment aims and tumor subtypes should be considered prior to recommending neoadjuvant therapy.
Certain comorbidities or poor performance status may preclude the safe administration of chemotherapy. Age and race may also predict response and may reflect differences in tumor biology. For example, women under the age of 35 years respond better than older women to neoadjuvant chemotherapy.[9] In a large retrospective study reported by M.D. Anderson Cancer Center investigators, race did not predict differences in pCR rate or breast cancer outcomes in women with triple-negative disease receiving neoadjuvant chemotherapy.[10] However, in a smaller single-institution analysis, African-American women with triple-negative breast cancer were less likely than Caucasian or other women with similar tumor characteristics to achieve a pCR.[11]
Neoadjuvant endocrine therapy is currently favored for patients with low-grade hormone receptor–positive breast cancer who are unlikely to respond to chemotherapy and those whose age or comorbidities preclude the administration of chemotherapy.[2]
Preoperative Assessment
Accurate clinical, radiologic, and pathologic evaluation of an individual’s breast cancer is imperative prior to initiating neoadjuvant chemotherapy. Clinical examination can estimate tumor size and presence of lymph node metastases. Baseline ultrasound of the affected breast and bilateral mammography should be performed. If breast conservation is considered, other imaging modalities, such as magnetic resonance imaging (MRI), may be employed, especially if results do not correlate appropriately with clinical findings or if tumors are lobular or multicentric in nature. Image-guided placement of a metal clip into the tumor bed is recommended, ideally at baseline, for women who may become candidates for BCT, as obtaining a pCR may otherwise preclude accurate identification of the original tumor site. A diagnostic core biopsy is essential to determine histologic tumor characteristics, including ER, PR, and HER2 status.[2] Ultrasound-guided fine-needle aspiration (FNA) of the axilla may assist in baseline staging.[12] If the axilla is clinically negative and the FNA has not revealed micrometastases, sentinel lymph node biopsy (SLNB) should be considered to allow for proper axillary staging and determine optimal local therapy.[13]
Systemic Therapy
The majority of patients undergoing neoadjuvant therapy will receive chemotherapy with or without trastuzumab. The ideal duration of neoadjuvant therapy has not been established, mainly due to heterogeneity in clinical trials reported to date. Administration of the entire planned course of therapy over 4 to 6 months prior to surgery is usually recommended.[2] Neoadjuvant hormonal therapy for 3 to 6 months may be considered in postmenopausal women with hormone-responsive disease who may be unfit for or not likely to benefit from chemotherapy.[14]
Chemotherapy
Early neoadjuvant chemotherapy trials utilized CMF-like (cyclophosphamide, methotrexate, and fluorouracil [5-FU]) and anthracycline-based regimens.[15,16] One of the largest initial studies, conducted by investigators from the National Surgical Adjuvant Breast and Bowel Project (NSABP), trial B-18, included 1,523 women with primary operable breast cancer who were randomly assigned to four cycles of doxorubicin and cyclophosphamide (AC) every 3 weeks either prior to or following breast surgery. With 9 years of follow-up, there was no significant difference in overall survival among the two groups. However, women achieving a pCR had a 50% reduction in risk of death compared to the entire group (relative risk = 0.5; 95% confidence interval = 0.32–0.78).[4] Furthermore, BCT rates were significantly higher in women who received neoadjuvant therapy compared to those receiving adjuvant treatment (67% and 60%, respectively, P = .002).
The addition of a taxane to anthracycline-based neoadjuvant regimens has resulted in higher pCR rates and a survival benefit. In the Aberdeen trial, 162 patients with a complete or partial response to four cycles of an anthracycline-based regimen were randomized to four additional cycles of the same regimen or to four cycles of docetaxel (Taxotere). With a median follow-up of 65 months, women who were sequenced to docetaxel had an improved pCR rate compared to the anthracycline-treated women (34% and 16%, respectively, P = .04), an improvement that was correlated with an impressive overall survival benefit (95% for the docetaxel arm vs 78% for the standard arm, P = .04).[17] In the larger NSABP trial B-27 (N = 2,411), despite a doubling of the pCR rate from 13.7% to 26.1% with the addition of docetaxel to AC, a significant overall survival difference was not observed between the treatment arms. However, at 6.5 years of follow-up, patients achieving a pCR had improved disease-free survival (hazard ratio [HR] = 0.45, P < .0001) and overall survival (HR = 0.33, P < .0001), confirming that pCR can be used as a surrogate marker for an individual’s long-term prognosis.[18]
TABLE 1
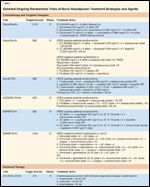
Selected Ongoing Randomized Trials of Novel Neoadjuvant Treatment Strategies and Agents
Subsequent and ongoing trials have attempted to improve further on these results by increasing treatment intensity-for example, by delivering chemotherapy in a dose-dense fashion, or by adding other chemotherapeutic agents to standard regimens. In the GeparDuo trial, participants received 8 weeks of dose-dense doxorubicin and docetaxel or 24 weeks of a sequential AC-followed-by-docetaxel approach. The rates of BCT and pCR were higher in the sequential arm but may reflect differences in the number of agents, cycles, and duration of therapy.[19] In another German study, 688 women with high-risk tumors (> 3 cm, inflammatory subtype) were randomly assigned to receive neoadjuvant sequential and dose-intensified epirubicin and paclitaxel every 2 weeks or concurrent epirubicin and paclitaxel every 3 weeks. All patients have also received three cycles of adjuvant CMF. Compared to the standard arm, women in the dose-dense arm had higher pCR rates and enjoyed improved disease-free (HR = 0.71; P = .011) and overall survival (HR = 0.83; P = .041).[20] Ongoing studies are investigating the addition of newer agents such as capecitabine (Xeloda) or gemcitabine (Gemzar) to an anthracycline-taxane combination (Table 1).
HER2-Targeted Therapies
The addition of trastuzumab to cytotoxic therapy has led to a considerable reduction in breast cancer recurrence and death in the adjuvant setting. Several small phase II studies have reported impressive pCR rates when trastuzumab was added to neoadjuvant anthracycline and taxane-based regimens. Concurrent paclitaxel and trastuzumab, followed by concurrent FEC (5-FU, epirubicin, cyclophosphamide) and trastuzumab yielded an impressive pCR rate of 60%, without an increase in cardiac toxicity compared to chemotherapy alone.[21] A non–anthracycline-based approach combining carboplatin, weekly paclitaxel, and trastuzumab was associated with a pCR rate of 76%.[22] In the phase III NeOAdjuvant Herceptin (NOAH) trial, the addition of trastuzumab to three cycles of doxorubicin and paclitaxel, followed by four cycles of paclitaxel and three cycles of CMF was associated with an improvement in overall response rate compared to chemotherapy alone (81% vs 73%, P = .18) and pCR rate (43% vs 23%, P = .002).[23]
Based on results available to date, trastuzumab should be used as a component of the neoadjuvant treatment strategy for women with HER2-positive early breast cancer. Large ongoing studies will help clarify the optimal use of trastuzumab-based regimens or the incorporation of other HER2-targeted therapies such as lapatinib (Tykerb) in the neoadjuvant setting (Table 1).
Hormonal Therapy
Neoadjuvant hormonal therapy is currently recommended for postmenopausal women with hormone-responsive disease who have contraindications to or are too frail to receive chemotherapy.[2] Although the toxicity profile of neoadjuvant hormonal therapy over the course of 3 to 6 months is favorable, the pCR rates obtained (1%–8%) are far lower than has been reported with chemotherapy.[24] Despite the low pCR rates obtained overall with neoadjuvant hormonal therapy, the benefit obtained from neoadjuvant chemotherapy may be no better for women with highly hormone-responsive disease. In a phase II study comparing 3 months of anastrozole (Arimidex) or exemestane (Aromasin) vs 4 cycles of doxorubicin and paclitaxel, the overall response rate was similar for both treatment modalities with higher BCT rates in the endocrine-treated patients (33% vs 24%).[25]
Neoadjuvant tamoxifen is associated with an overall response rate of 33%, with maximum response occurring after up to 12 months of therapy in some patients.[14] More recently, the aromatase inhibitors (AIs) have been compared to tamoxifen in the neoadjuvant setting. In most studies that randomized patients to 4 months of neoadjuvant AI or tamoxifen, overall objective response and BCT rates were either statistically significantly improved in the AI-treated women[24] or comparable to tamoxifen-associated outcomes.[26,27] Whether specific tumor characteristics such as HER2 status are more predictive for response to one vs another hormonal agent is not known. An American College of Surgeons Oncology Group (ACOSOG) trial is currently comparing the efficacy of anastrozole, letrozole (Femara), and exemestane in the neoadjuvant setting (Table 1). Whether there is a subset of premenopausal women with hormone-responsive breast cancer who would derive benefit from hormonal therapy over chemotherapy remains to be determined. In a small investigation of ovarian suppression and an AI in this population, the pCR rate was 3%.[28]
Novel Treatment Strategies
Many novel targeted therapies are currently under investigation in an effort to further improve the efficacy of neoadjuvant therapy. Since studies in the adjuvant setting require thousands of women and long-term follow-up, the neoadjuvant setting has been utilized to determine potential activity of novel agents.
Bevacizumab (Avastin), a monoclonal antibody to vascular endothelial growth factor (VEGF), in combination with a cytotoxic agent such as paclitaxel, represents a common standard first-line therapy for metastatic breast cancer and is under investigation in the adjuvant setting. The addition of bevacizumab to neoadjuvant chemotherapy has yielded promising results. Phase II trials to date have indicated a pCR rate as high as 42% in patients receiving concurrent neoadjuvant bevacizumab and chemotherapy, with an acceptable toxicity profile.[29,30] Six cycles of bevacizumab, docetaxel, and capecitabine resulted in a pCR rate of 22% and overall clinical response rate of 72% in a HER2-negative population.[31] However, the combination of bevacizumab and a taxane in patients with inflammatory breast cancer and locally advanced disease has not been associated with pCR.[32] Ongoing large trials are further evaluating bevacizumab and other promising agents with combination chemotherapy (Table 1).
Another interesting area of investigation is the addition of targeted therapies to hormonal agents in the neoadjuvant setting, in an effort to overcome endocrine resistance and improve response to therapy. The neoadjuvant setting can also be used to test other hypotheses in women who receive standard therapy. For example, preclinical and clinical evidence support a role for insulin and associated growth factors in the pathogenesis of breast cancer and disease outcomes. In a retrospective analysis of diabetic patients receiving neoadjuvant chemotherapy, those receiving metformin-an oral hypoglycemic agent that increases insulin sensitivity and reduces insulin levels in patients with diabetes-had a pCR rate of 24% (n = 68) compared to 8% in patients who were not receiving metformin (n = 87).[33] These interesting observations warrant further evaluation in a prospective study.
Assessment of Response to Therapy
Regular assessment of response to therapy by the treating oncologist and the surgical team is critical. Women with a response to treatment or with stable disease should continue the prescribed therapy. Women with progressive disease should be transitioned to a non–cross-resistant regimen or proceed with a surgical intervention for the operable disease. Evaluation of response to therapy is also required to determine the optimal surgical interventions, especially when BCT is desired.
Several trials have investigated a switch in chemotherapeutic agents based on response to initial therapy. As noted, in the Aberdeen trial, a switch to a non–cross-resistant agent (docetaxel) in those responding to therapy resulted in an improvement in pCR and survival compared to those who continued on the initial anthracycline-based regimen.[17] In the GeparTrio trial, patients not responding to therapy after two cycles of docetaxel, doxorubicin, and cyclophopsphamide (TAC) were randomized to four further cycles of TAC or to vinorelbine and capecitabine (NX). The pCR rates were low in both arms (approximately 6%) with equal rates of BCT (approximately 60%), but the NX arm was better tolerated. For patients who responded to the first two cycles of TAC, treatment intensification to six vs four further cycles of TAC did not improve pCR rates (23.5% vs 21%, respectively, P = .27) and was associated with increased toxicity.[34,35] Although switching to a non–cross-resistant regimen is advised when a patient is progressing through chemotherapy, there is no clear evidence that this approach will improve breast cancer outcomes in women who respond to therapy. Further study is needed.
Surgical Considerations
While the administration of neoadjuvant therapy has resulted in enhancement of BCT,[3] some have raised concerns that the approach may be associated with higher locoregional recurrence rates, in particular when patients are initially candidates for mastectomy but subsequently undergo BCT. Overall, studies that evaluated locoregional recurrence rates suggest that with careful selection of patients for BCT, adequate treatment regimens, and negative margins on pathologic examination, local recurrence does not appear to be higher for those receiving neoadjuvant therapy vs those undergoing a primary surgical approach.[2] Mastectomy remains the surgical option of choice for certain patients-for example, when the extent of tumor downstaging does not permit BCT or for multicentric disease.
The optimal management of the axilla following neoadjuvant therapy has not been established. SLNB is now viewed as standard of care for patients with T1-2, N0 tumors.[36] Few studies have evaluated the feasibility and accuracy of SLNB prior to neoadjuvant therapy. In one small study, SLNB was performed in 25 women prior to initiating neoadjuvant therapy and was followed by axillary lymph node dissection (ALND) in those with a positive node. Fourteen patients (56%) did not require ALND.[13] Other potential advantages of pretreatment SLNB include the provision of additional prognostic information that may aid in treatment decision-making and the availability of untreated metastatic tissue for correlative studies. A disadvantage of performing SLNB prior to chemotherapy, however, is that a positive result will necessitate a second axillary procedure after chemotherapy.
The accuracy of SLNB following neoadjuvant therapy has been investigated prospectively in several small studies that have reported false-negative rates ranging from 10% to 33% for SLNB in this setting.[37] A recent prospective study, however, indicates that SLNB after neoadjuvant chemotherapy is feasible with equal sentinel node detection rates, false-negative rates (11.5%), and accuracy, compared to results from a pooled analysis of patients undergoing SLNB in the absence of neoadjuvant chemotherapy.[38] A large retrospective analysis also indicates that SLNB is as accurate for axillary staging after chemotherapy as it is prior to chemotherapy.[39] Axillary dissection is not routinely performed where SLNB is negative after neoadjuvant chemotherapy, although randomized data are not available to support this approach. Taken together, data available to date suggest that SLNB may be performed either prior to or following neoadjuvant therapy, but the comparative effects on long-term disease outcome are yet to be defined.
Pathologic Considerations
Once the primary tumor and lymph node specimens are available, accurate histopathologic assessment is critical. Clear communication relating to specific clinical information between the surgical oncologist and the pathologist is essential. The pathologist must assess response to therapy, patterns of tumor shrinkage, and margin status, emphasizing the need for experience. Standardized definitions of pCR should be employed, ideally indicating no residual invasive and noninvasive tumor cells in the breast and in the axillary nodes. Other definitions are available to determine response to treatment. For example, residual cancer burden (RCB) is a continuous index that combines pathologic measurements of primary tumor (size and cellularity) and nodal metastases (number and size) and may more accurately predict outcomes for individuals.[40] Documentation of the residual stage should include ypTNM classification to alert the reader that the patient received systemic therapy prior to the definitive breast surgery.[41]
Reference Guide
Therapeutic Agents
Mentioned in This Article
Anastrozole (Arimidex)
Bevacizumab (Avastin)
Capecitabine (Xeloda)
Carboplatin
Cyclophosphamide
Docetaxel (Taxotere)
Doxorubicin
Epirubicin
Exemestane (Aromasin)
Fluorouracil (5-FU)
Gemcitabine (Gemzar)
Lapatinib (Tykerb)
Letrozole (Femara)
Methotrexate
Paclitaxel
Trastuzumab (Herceptin)
Vinorelbine
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Adjuvant Therapy
As it is advised to administer the entire course of recommended chemotherapy prior to surgery, there is currently no clear role for adjuvant chemotherapy even when a pCR is not obtained. Radiation therapy following BCT should be administered to women who have received neoadjuvant therapy to reduce the risk of local recurrence. Decisions regarding postmastectomy radiation in women who received neoadjuvant therapy should be based on baseline clinical extent of disease and pathologic extent of residual disease. Chest wall and regional nodal radiation should be considered for women with baseline clinical stage III disease or for those with histologically positive lymph nodes following the neoadjuvant treatment. The role of postmastectomy radiation in women with clinical stage II disease who have negative lymph nodes following chemotherapy is under investigation.[42] Patients with hormone-responsive breast cancer should receive 5 or more years of adjuvant hormonal therapy with tamoxifen or an aromatase inhibitor per standard guidelines.[43] Maintenance trastuzumab should be continued for a total of 1 year in those with HER2-positive disease, given the clear benefits of this approach in the adjuvant setting.
Predictive Biomarkers
As we enter an era of “personalized” cancer treatment, the preoperative period has been recognized as ideal for evaluating surrogate biomarkers for the prediction of response to therapy and clinical outcome. Valuable information can be obtained using small numbers of patients in a short time frame, by assessing tumor response to therapy in vivo and measuring biochemical or radiologic changes in malignant tissue prior to, during, and following neoadjuvant therapy. Standard clinicopathologic factors such as age, receptor status, grade, and proliferation index are already a component of routine clinical practice in determining the choice of therapy for those with early breast cancer.
In addition to single genes or proteins, gene-expression profiling allows for the rapid assessment of multiple genes rather than single genes using high-throughput DNA sequencing and may be used to predict both response to therapy and clinical outcome. Several trials have evaluated multigene assays as predictors of response to therapy in the preoperative setting, and validation efforts are ongoing.[44,45] Host genetic factors such as BRCA1 or BRCA2 mutation status may also play a role in predicting response to neoadjuvant chemotherapy and are under investigation.
Imaging techniques may also provide early information regarding tumor response. MRI correctly predicts residual tumor in 63% of cases, followed in order of sensitivity by clinical examination, ultrasound, and mammography.[46] Positron-emission tomography (PET) may allow for early prediction for pathologic response 2 to 3 months post commencement of systemic therapy. In a prospective trial of 64 patients with early breast cancer, a decrease in the standardized uptake value (SUV) of 18F-fluorodeoxyglucose uptake with PET was compared with the pathologic response. The sensitivity, specificity, and negative predictive value of PET were 89%, 95%, and 85% after two courses, and 88%, 73%, and 83% after three courses, respectively.[47] Reports indicate that combining MRI and PET for response evaluation may provide valuable information, but given the expense associated with these modalities, require further study.
Conclusions
TABLE 2

Key Points in the Neoadjuvant Approach to Patients With Primary Operable Breast Cancer
Neoadjuvant therapy is recommended to women with locally advanced breast cancer and, in recent years, to many women with primary operable stage II or III disease. Prior to initiating neoadjuvant therapy, it is important to determine the aims of treatment, and to carefully assess both patient and tumor characteristics to determine appropriate candidates for this therapeutic approach and the choice of systemic treatment. A multidisciplinary assessment at baseline and during treatment is essential to determine tumor characteristics, to evaluate response, and to determine the most appropriate local therapy (Table 2).
Ongoing trials aim to improve breast cancer outcomes by investigating novel chemotherapeutic combinations and schedules, the addition of targeted therapies to standard regimens, and drug mechanisms of action. Other investigations attempt to determine optimal management of the axilla and selective use of radiation therapy. As we enter an era that strives to provide individualized therapy, the identification of clinically relevant predictive and prognostic biomarkers are required to aid treatment decisions in order to maximize the efficacy and minimize treatment-related toxicity. Future studies should take advantage of the unique opportunity that the neoadjuvant setting provides as an in vivo model to understand not only drug mechanisms of action but also patterns of sensitivity and resistance to systemic therapy.
Financial Disclosure: Dr. Stearns has received investigator-initiated grants from Abraxis, Merck, Novartis, and Pfizer, and has received honoraria from AstraZeneca.
Acknowledgments:The authors thank their colleagues at the Breast Cancer Program at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins for many helpful discussions over the years.
References:
References
1. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365:1687-1717, 2005.
2. Kaufmann M, von Minckwitz G, Bear HD, et al: Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: New perspectives 2006. Ann Oncol 18:1927-1934, 2007.
3. Mauri D, Pavlidis N, Ioannidis JP: Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst 97:188-194, 2005.
4. Wolmark N, Wang J, Mamounas E, et al: Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr (30):96-102, 2001.
5. Colleoni M, Viale G, Zahrieh D, et al: Expression of ER, PgR, HER1, HER2, and response: A study of preoperative chemotherapy. Ann Oncol 19:465-472, 2008.
6. Rody A, Karn T, Solbach C, et al: The erbB2+ cluster of the intrinsic gene set predicts tumor response of breast cancer patients receiving neoadjuvant chemotherapy with docetaxel, doxorubicin and cyclophosphamide within the GEPARTRIO trial. Breast 16:235-240, 2007.
7. Nakano E, Hojo T, Masumara K: The response to neoadjuvant chemotherapy and prognosis of triple-negative breast cancer patients (abstract 5121). Presented at the San Antonio Breast Cancer Symposium; San Antonio, Texas; December 10-14, 2008.
8. Colleoni M, Viale G, Zahrieh D, et al: Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: A study of preoperative treatment. Clin Cancer Res 10:6622-6628, 2004.
9. Braud AC, Asselain B, Scholl S, et al: Neoadjuvant chemotherapy in young breast cancer patients: Correlation between response and relapse? Eur J Cancer 35:392-397, 1999.
10. Dawood S, Broglio K, Kau SW, et al: Triple receptor-negative breast cancer: The effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol 27:220-226, 2009.
11. Balmanoukian A, Zhang Z, Jeter S, et al: African American women who receive primary anthracycline- and taxane-based chemotherapy for triple-negative breast cancer suffer worse outcomes compared with white women. J Clin Oncol 27:e35-37; author reply e38-39, 2009.
12. Kilbride KE, Lee MC, Nees AV, et al: Axillary staging prior to neoadjuvant chemotherapy for breast cancer: Predictors of recurrence. Ann Surg Oncol 15:3252-3258, 2008.
13. van Rijk MC, Nieweg OE, Rutgers EJ, et al: Sentinel node biopsy before neoadjuvant chemotherapy spares breast cancer patients axillary lymph node dissection. Ann Surg Oncol 13:475-479, 2006.
14. Preece PE, Wood RA, Mackie CR, et al: Tamoxifen as initial sole treatment of localised breast cancer in elderly women: A pilot study. Br Med J (Clin Res Ed) 284:869-870, 1982.
15. Ragaz J, Baird R, Rebbeck P, et al: Neoadjuvant (preoperative) chemotherapy for breast cancer. Cancer 56:719-724, 1985.
16. Swain SM, Sorace RA, Bagley CS, et al: Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res 47:3889-3894, 1987.
17. Heys SD, Sarkar T, Hutcheon AW: Primary docetaxel chemotherapy in patients with breast cancer: Impact on response and survival. Breast Cancer Res Treat 90:169-185, 2005.
18. Bear HD, Anderson S, Smith RE, et al: Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project protocol B-27. J Clin Oncol 24:2019-2027, 2006.
19. von Minckwitz G, Raab G, Caputo A, et al: Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: The GEPARDUO study of the German Breast Group. J Clin Oncol 23:2676-2685, 2005.
20. Untch M, Mobus V, Kuhn W, et al: Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol 27:2938-2945, 2009.
21. Buzdar AU, Valero V, Ibrahim NK, et al: Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: An update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 13:228-233, 2007.
22. Sikov WM, Dizon DS, Strenger R, et al: Frequent pathologic complete responses in aggressive stages II to III breast cancers with every-4-week carboplatin and weekly paclitaxel with or without trastuzumab: A Brown University Oncology Group study. J Clin Oncol 27:4693-4700, 2009.
23. Gianni L, Semiglazov V, Manikhas G: Neoadjuvant trastuzumab in locally advanced breast cancer (NOAH): Antitumour and safety analysis (abstract 532). J Clin Oncol 25(18S):10s, 2007.
24. Eiermann W, Paepke S, Appfelstaedt J, et al: Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 12:1527-1532, 2001.
25. Semiglazov VF, Semiglazov VV, Dashian GA, et al: [Phase II clinical trial of neoadjuvant hormone therapy in comparison with chemotherapy of patients with breast cancer]. Vopr Onkol 53:400-408, 2007.
26. Cataliotti L, Buzdar AU, Noguchi S, et al: Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: The Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trial. Cancer 106:2095-2103, 2006.
27. Smith IE, Dowsett M, Ebbs SR, et al: Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 23:5108-5116, 2005.
28. Torrisi R, Bagnardi V, Pruneri G, et al: Antitumour and biological effects of letrozole and GnRH analogue as primary therapy in premenopausal women with ER and PgR positive locally advanced operable breast cancer. Br J Cancer 97:802-808, 2007.
29. Makhoul I, Vicki KS, Korourian S: Bevacizumab combined with chemotherapy significantly improves pathological complete response in patients with operable or locally advanced breast cancer (abstract 5114). Presented at the San Antonio Breast Cancer Symposium; San Antonio, Texas; December 9-13, 2008.
30. Waintraub SE, Tuchman V, Hackensack NJ: The role of preoperative neoadjuvant cytoreductive dose-dense bevacizumab plus docetaxel followed by bevacizumab-doxorubicin-cyclophosphamide regimen in locally advanced operable breast cancer (abstract e11524). J Clin Oncol 27(suppl), 2009.
31. Greil R, Moik M, Reitsamer R, et al: Neoadjuvant bevacizumab, docetaxel and capecitabine combination therapy for HER2/neu-negative invasive breast cancer: Efficacy and safety in a phase II pilot study. Eur J Surg Oncol 2009;35:1048-1054.
32. Wedam SB, Low JA, Yang SX, et al: Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol 24:769-777, 2006.
33. Jiralerspong S, Palla SL, Giordano SH, et al: Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 27:3297-3302, 2009.
34. von Minckwitz G, Kummel S, Vogel P, et al: Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: Phase III randomized GeparTrio trial. J Natl Cancer Inst 100:542-551, 2008.
34. von Minckwitz G, Kummel S, Vogel P, et al: Intensified neoadjuvant chemotherapy in early-responding breast cancer: Phase III randomized GeparTrio study. J Natl Cancer Inst 100:552-562, 2008.
36. Lyman GH, Giuliano AE, Somerfield MR, et al: American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 23:7703-7720, 2005.
37. Nason KS, Anderson BO, Byrd DR, et al: Increased false negative sentinel node biopsy rates after preoperative chemotherapy for invasive breast carcinoma. Cancer 89:2187-2194, 2000.
38. Classe JM, Bordes V, Campion L, et al: Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: Results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol 27:726-732, 2009.
39. Hunt KK, Yi M, Mittendorf EA, et al: Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg Aug 27, 2009 (epub ahead of print).
40. Symmans WF, Peintinger F, Hatzis C, et al: Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25:4414-4422, 2007.
41. Kaufmann M, Hortobagyi GN, Goldhirsch A, et al: Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: An update. J Clin Oncol 24:1940-1949, 2006.
42. Buchholz TA, Lehman CD, Harris JR, et al: Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: A National Cancer Institute conference. J Clin Oncol 26:791-797, 2008.
43. Buzdar AU, Coombes RC, Goss PE, et al: Summary of aromatase inhibitor clinical trials in postmenopausal women with early breast cancer. Cancer 112:700-709, 2008.
44. Gianni L, Zambetti M, Clark K, et al: Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol 23:7265-7277, 2005.
45. Ayers M, Symmans WF, Stec J, et al: Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. J Clin Oncol 22:2284-2293, 2004.
46. Balu-Maestro C, Chapellier C, Bleuse A, et al: Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat 72:145-152, 2002.
47. Rousseau C, Devillers A, Sagan C, et al: Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol 24:5366-5372, 2006.