Myalgias and Arthralgias Associated With Paclitaxel
Paclitaxel-induced myalgias and arthralgias occur in a significantfraction of patients receiving therapy with this taxane, potentiallyimpairing physical function and quality of life. Paclitaxel-inducedmyalgias and arthralgias are related to individual doses; associationswith the cumulative dose and infusion duration are less clear. Identificationof risk factors for myalgias and arthralgias could distinguisha group of patients at greater risk, leading to minimization of myalgiasand arthralgias through the use of preventive therapies. Optimalpharmacologic treatment and possibilities for the prevention of myalgiasand arthralgias associated with paclitaxel are unclear, partially dueto the small number of patients treated with any one medication. Theeffectiveness of nonsteroidal anti-inflammatory drugs (NSAIDs) is themost frequently documented pharmacologic intervention, although noclear choice exists for patients who fail to respond to NSAIDs. However,the increasing use of weekly paclitaxel could necessitate daily administrationof NSAIDs for myalgias and arthralgias and leave patients at riskfor adverse effects. This concern may also limit the use of corticosteroidsfor the prevention and treatment of paclitaxel-induced myalgias andarthralgias. Data from case reports suggest that gabapentin (Neurontin),glutamine, and, potentially, antihistamines (eg, fexofenadine [Allegra])could be used to treat and/or prevent myalgias and arthralgias. Giventhe safety profile of these medications, considerable enthusiasm existsfor evaluating their effectiveness in the prevention and treatment ofpaclitaxel myalgias and arthralgias, particularly in the setting ofweekly paclitaxel administration.
ABSTRACT: Paclitaxel-induced myalgias and arthralgias occur in a significant fraction of patients receiving therapy with this taxane, potentially impairing physical function and quality of life. Paclitaxel-induced myalgias and arthralgias are related to individual doses; associations with the cumulative dose and infusion duration are less clear. Identification of risk factors for myalgias and arthralgias could distinguish a group of patients at greater risk, leading to minimization of myalgias and arthralgias through the use of preventive therapies. Optimal pharmacologic treatment and possibilities for the prevention of myalgias and arthralgias associated with paclitaxel are unclear, partially due to the small number of patients treated with any one medication. The effectiveness of nonsteroidal anti-inflammatory drugs (NSAIDs) is the most frequently documented pharmacologic intervention, although no clear choice exists for patients who fail to respond to NSAIDs. However, the increasing use of weekly paclitaxel could necessitate daily administration of NSAIDs for myalgias and arthralgias and leave patients at risk for adverse effects. This concern may also limit the use of corticosteroids for the prevention and treatment of paclitaxel-induced myalgias and arthralgias. Data from case reports suggest that gabapentin (Neurontin), glutamine, and, potentially, antihistamines (eg, fexofenadine [Allegra]) could be used to treat and/or prevent myalgias and arthralgias. Given the safety profile of these medications, considerable enthusiasm exists for evaluating their effectiveness in the prevention and treatment of paclitaxel myalgias and arthralgias, particularly in the setting of weekly paclitaxel administration.
TABLE 1

Role of Paclitaxel in Cancer Treatment
Amelioration of the side effects of paclitaxel is essential, given its effectiveness in the treatment of several malignancies (Table 1). Historically, the dose-limiting toxicities of paclitaxel administered as a 24-hour infusion were hypersensitivity reactions and neutropenia, which are controlled largely through the use of systemic premedications and hematopoietic growth factors. Paclitaxel is also administered as a 1-or 3-hour infusion, with weekly administration recently gaining considerable popularity. Neuromuscular toxicities are frequently dose-limiting with 1-or 3-hour infusions.[1-3] The neurotoxicities of paclitaxel include sensory neuropathy, motor neuropathy, autonomic neuropathy, myopathy (ie, myalgias/arthralgias), and central nervous system effects.[4]
Although sensory neuropathy is perceived to be the most common adverse effect, the incidence of myalgias and arthralgias closely approximates[ 5-9] or surpasses[10-13] that of sensory neuropathy. A relation ship between myalgias/arthralgias and subsequent sensory neuropathy has not been conclusively shown, but myalgias and arthralgias may be an initial symptomatic manifestation of peripheral nerve injury.[8]
Transient myalgias and arthralgias begin 24 to 72 hours after paclitaxel administration and usually resolve in 4 to 7 days. The anatomic distribution of myalgias and arthralgias is variable, with some patients experiencing diffuse myalgias and arthralgias, and others experiencing such effects localized to large axial muscles (eg, shoulder and paraspinal muscles) or lower extremities.[4,14] There is considerable variability in the reported incidence of paclitaxel-induced myalgias and arthralgias,[5-7,9-12,15,16] potentially due to discrepancies between assessment methods and toxicity grading (Table 2).[6,9,17]
This manuscript reviews the published literature on the incidence and severity of paclitaxel-associated myalgias and arthralgias, as well as the effectiveness of medications for these adverse effects.
Pathophysiology
TABLE 2

Examples of Categoric Assignment of Myalgias/Athralgias
The pathophysiology of paclitaxelinduced neurotoxicity has been evaluated in animal models, which suggest a supportable hypothesis for the mech- anism causing sensory neuropathy. Microtubules are important in the development and maintenance of neurons, with microtubule elongation contributing to the growth of neurites. In the mature axon, microtubules have a significant role in mediating axonal transport and provide structural support for neurons. Paclitaxel is cytotoxic through polymerization and subsequent stabilization of microtubule bundles, and these processes could be expected to affect neuronal development and function.[18]
Animal studies support this hypothesis, documenting microtubule aggregation in cultured mouse dorsal root-ganglion cells and rat Schwann cells and axons, with subsequent demyelination and loss of axoplasmic transport.[19-22] The regenerative response of axons and Schwann cells to nerve crush injuries is inhibited in rodents who have received paclitaxel.[ 23,24] It is undetermined whether myalgias and arthralgias are produced by a similar pathophysiologic process.
Risk Factors
Several studies have been undertaken to identify risk factors for myalgias and arthralgias, with the hope of identifying a paclitaxel dose or infusion duration that minimizes these adverse effects.[25] To date, no correlation has been observed between myalgias/arthralgias and patient parameters such as age, sex, height, prior chemotherapy, renal or hepatic function, or sites of metastases.[25] Concurrent signs of inflammation and elevations in muscle enzyme concentrations, such as creatinine phosphokinase, have not been noted.[4]
No relationship has been found between myalgias/arthralgias and the pharmacokinetic characteristics of paclitaxel (ie, peak plasma concentration, area under the plasma concentration vs time curve, duration of paclitaxel concentration > 0.1 μM).[25] Moreover, concurrent administration of cisplatin, which is known for its neurotoxicity, does not appear to affect the severity of myalgias and arthralgias.[ 4,26] The relationships between paclitaxel-induced myalgias and arthralgias and factors such as single dose, cumulative dose, and duration of infusion have all been evaluated.[3,6,8,9,11,14,16,25,27,28]
Single Dose
Increasing single doses of paclitaxel are positively correlated with the incidence and severity of myalgias and arthralgias. Mild and self-limiting myalgias and arthralgias usually occur at doses less than 170 mg/m2. Initial data from case series and phase I/II trials suggested that myalgias and arthralgias are more common at doses greater than 190 mg/m2.[4,8,25,27,28]
The incidence of myalgias and arthralgias of any grade increased from 54% to 67% in 391 patients with ovarian cancer who received paclitaxel at 135 mg/m2 or 175 mg/m2, respectively (P = .01).[11] Eisenhauer et al designed this study such that women were randomized in a bifactorial design to receive either 135 mg/m2 or 175 mg/m2 as a 3-hour or 24-hour infusion, leading to the creation of four treatment groups.[11]
In 599 patients with metastatic non-small-cell lung cancer (NSCLC), grade 3/4 myalgias and arthralgias occurred in 1% of those receiving paclitaxel at 135 mg/m2 IV (over 24 hours) with cisplatin, compared to 7% of those receiving paclitaxel at 250 mg/m2 IV (over 24 hours), cisplatin, and granulocyte colonystimulating factor (G-CSF, Neupogen).[ 27] The incidence of myalgias and arthralgias of any grade was 23% and 14% in 198 patients with NSCLC who received carboplatin (Paraplatin) with a 3-hour infusion of paclitaxel, 175 mg/m2 or 225 mg/m2, respectively.[ 29] The difference in incidence of myalgias and arthralgias between these two groups was not statistically significant (P > .05).[29]
Thus, available data suggest a higher incidence and severity of myalgias and arthralgias when higher single doses of paclitaxel are administered, with the incidence rising at single paclitaxel doses greater than or equal to 135 mg/m2.
Cumulative Dose
The relationship between paclitaxel- induced myalgias and arthralgias and cumulative dose is unclear. A retrospective analysis of neuromuscular toxicity in 247 patients treated with paclitaxel revealed a Spearman's correlation coefficient of 0.218 (P = .0006) for myalgias and arthralgias and total paclitaxel dose.[25] A wide range of cumulative paclitaxel doses (median: 630 mg/m2, range: 35 to 3,150 mg/m2) were administered over a 3-hour infusion every 3 weeks.[25] Of the 85 patients who experienced grade 2 or 3 myalgias and arthralgias, 80 (94%) had at least grade 1 and 68 (80%) had at least grade 2 symptoms after the first course, indicating that myalgias and arthralgias occur during early treatment cycles in most patients who suffered more severe myalgias and arthralgias.
A case series in 27 patients re- ported a similar pattern in those who experienced sensory neuropathy following paclitaxel administration (135-300 mg/m2).[30] Although data from Kunitoh et al suggests a weak association between cumulative paclitaxel dose and myalgias and arthralgias, these adverse effects also have an early onset, suggesting that the total dose is not a critical risk factor for developing myalgias and arthralgias.
Infusion Duration
At equivalent paclitaxel doses, the length of infusion does not appear to relate to the incidence of myalgias and arthralgias,[6,11] despite initial reports suggesting that shorter infusions (ie, 1 to 3 hours vs 24 hours) led to an increased risk.[9] Eisenhauer et al reported that the overall incidence of myalgias and arthralgias was 64% with a 3-hour infusion vs 58% with a 24-hour infusion in 391 ovarian cancer patients who were randomized to one of four arms (ie, 135 mg/m2 3-hour infusion, 135 mg/m2 24-hour infusion, 175 mg/m2 3-hour infusion, 175 mg/m2 24-hour infusion). The incidence was collapsed across doses.[11]
Similar findings were observed in 557 patients with breast cancer who participated in the National Surgical Adjuvant Breast and Bowl Project (NSABP) B-26 study, which was a randomized, controlled trial of 3-hour or 24-hour infusion of paclitaxel, 250 mg/m2.[6] Myalgias and arthralgias occurred in 82% of patients who received a 3-hour infusion and in 87% of those receiving a 24-hour infusion.[ 6] The incidence of grade 3/4 myalgias and arthralgias (19% in both groups) was not affected by length of infusion.
No published trials have compared 1-hour paclitaxel infusions with 3-or 24-hour infusions. A phase II trial of 100 patients with NSCLC who received paclitaxel, 225 mg/m2 over 1 hour, followed by carboplatin suggests that the incidence and severity of myalgias and arthralgias is similar to that seen with 3-and 24-hour infusions.[ 3] Of 94 evaluable patients, 63% experienced grade 1/2 myalgias and arthralgias and 5% had grade 3/4 symptoms. These results suggest that the length of paclitaxel infusion is not associated with significant differences in the incidence of myalgias and arthralgias; however, 1-hour infusion data are limited. Prospective trials gaining additional information about these potential risk factors and identifying novel risk factors are needed.
Pharmacologic Management
There is a paucity of data on the prevention and treatment of myalgias and arthralgias caused by paclitaxel. To date, no randomized, controlled trial for the management of this important adverse effect has been published. The effectiveness of pharmacologic therapies for the prevention and treatment of myalgias and arthralgias has been gleaned from case series or toxicity results of phase I-III clinical trials, with the majority of the published data being reported qualitatively. Medications that have been evaluated in this setting include nonsteroidal anti-inflammatory drugs (NSAIDs), opioid analgesics, corticosteroids, antihistamines, tricyclic antidepressants, intranasal calcitonin (Miacalcin), gabapentin (Neurontin), and glutamine (Table 3).
Treatment
TABLE 3
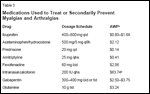
Medications Used to Treat or Secondarily Prevent Myalgias and Arthralgias
In the treatment setting, medications are used as needed when a patient experiences myalgias and arthralgias.
• NSAIDs-NSAIDs are the most extensively prescribed treatment for paclitaxel-induced myalgias and arthralgias.[3,4,6,12,31-37] Most data suggest that NSAIDs (eg, ibuprofen, 200-400 mg orally every 4-6 hours) yield partial to complete relief[ 5,8,13,31,32] or relief in the majority of patients,[1,33,34] although myalgias and arthralgias can be refractory to NSAIDs.[1,35] Quantitative data regarding efficacy are not available. While the use of NSAIDs is plausible for the treatment of myalgias and arthralgias, the increasing use of weekly paclitaxel could lead to continuous NSAID therapy, and the adverse effects of daily NSAID use (eg, platelet toxicity, renal dysfunction) are of concern, particularly in patients with cancer.
• Opioids-Opioids are anecdotally reported to treat myalgias and arthralgias refractory to NSAIDs, although data of their effectiveness are limited. Occasionally used in conjunction with acetaminophen, opioids partially or completely relieved paclitaxel- induced myalgias and arthralgias in one report.[8] Holmes and colleagues also noted that propoxyphene (65-130 mg orally every 3 hours) provided relief from myalgias and arthralgias when used alone or in combination with opioids, tricyclic antidepressants, or NSAIDs.[8] The effectiveness of opioids in this setting has not been qualitatively or quantitatively reported in other trials or case series.[6,9,33,35,36]
• Corticosteroids-Short-term administration (2-5 days) of corticosteroids (eg, prednisone, dexamethasone) has been shown to relieve paclitaxel-induced myalgias and arthralgias.[ 1,6,9,35,37-39] Corticosteroids have been used primarily to prevent myalgias and arthralgias during subsequent paclitaxel cycles. Available qualitative data suggest only partial[1] or no[9] resolution of myalgias and arthralgias with corticosteroid treatment. Although some of these results appear promising, the small number of patients as well as the lack of quantitative efficacy data do not provide conclusive evidence for the effectiveness of corticosteroids in myalgias and arthralgias treatment. The long-term adverse effects of utilizing 3 to 5 days of corticosteroids with weekly paclitaxel are also of concern.
• Antihistamine-Antihistamines may relieve paclitaxel-induced myalgias and arthralgias[6,40] and are an attractive option due to their possibly low side-effect profile, although more data are needed. Interest in their use emerged from the report of a patient who experienced complete pain relief from self-medication with 50 mg of mebhydroline (an antihistamine not available in the United States) for "painful pruritis" after paclitaxel administration.[40] Subsequent patients received oral terfenadine (Seldane), 60 mg twice daily, at the onset of myalgias and arthralgias. Of the five who received terfenadine, three experienced complete pain relief within 1 hour (two of whom were refractory to NSAIDs), one patient had partial relief, and one patient had no relief. Prophylactic use of terfenadine was unsuccessful.[40]
Terfenadine was the first-line treatment of myalgias and arthralgias in an NSABP B-26 trial of patients with breast cancer randomized to receive paclitaxel, 250 mg/m2 as a 3-or 24-hour infusion, although the effectiveness of terfenadine was not specifically noted.[6] Regardless, terfenadine was withdrawn from the US market in 1997, although its active metabolite, fexofenadine (Allegra), is commercially available.
• Other Agents-Other attempts to alleviate myalgias and arthralgias have been reported with tricyclic antidepressants and intranasal calcitonin. Tricyclic antidepressants have been reported to relieve paclitaxel-induced myalgias and arthralgias, although quantitative results are not available.[8] Intranasal calcitonin has been reported to relieve muscular and joint pain in five of eight breast cancer patients who experienced grade 3 myalgias and arthralgias refractory to NSAIDs or opioids after treatment with adjuvant paclitaxel or docetaxel (Taxotere).[41]
Prevention
Patients who have experienced myalgias and arthralgias with prior paclitaxel treatment may take a medication 24 hours following subsequent paclitaxel doses, regardless of the onset of such pain, with the hope of completely preventing or reducing its severity. Prevention of paclitaxel-induced myalgias and arthralgias is desirable because of the frequency of these complications, their sequelae, and variable efficacy in treating them. The majority of the data in the preventive setting is with corticosteroids, although efficacy with propoxyphene, gabapentin, and glutamine has also been reported.[1,6,9,31,36,38,42]
• Corticosteroids-Prophylactic use of prednisone has been evaluated in patients with advanced cancer who received 210, 250, or 300 mg/m2 over 3 hours every 3 weeks, with concurrent G-CSF in 35 patients.[9] After the first paclitaxel dose, 26 of 35 patients (74%) experienced grade 1-3 myalgias and arthralgias, which were treated with opioid analgesics. Oral prednisone, 40 mg/d for 2 to 5 days, was empirically administered in 10 patients, all of whom reported a prompt reduction in their discomfort.[9]
Markman and colleagues published a case series of approximately 300 ovarian cancer patients treated with paclitaxel-containing chemotherapy regimens, 46 of whom received prophylactic prednisone because their myalgias and arthralgias were unrelieved by ibuprofen.[38] Oral prednisone, 10 mg twice daily, starting 24 hours after the completion of chemotherapy and continuing for 5 days (alone or in combination with NSAIDs), prevented myalgias and arthralgias in 39 (85%) of the 46 women. Prophylactic use of oral dexamethasone (4-8 mg twice daily for 4 days) reduced grade 3 myalgias and arthralgias in four patients receiving combination carboplatin/paclitaxel.[ 1] However, these promising preliminary data are tempered by the risks of daily corticosteroid administration, especially with weekly paclitaxel administration.
• Propoxyphene and Gabapentin-Limited data are available on propoxyphene and gabapentin for the prevention of paclitaxel-induced my- algias and arthralgias. Sarris and associates reported that propoxyphene (dose not specified) prevented myalgias and arthralgias in patients with non-Hodgkin's lymphoma.[36]
One case report documents the effectiveness of gabapentin in preventing taxane-induced myalgias and arthralgias in two patients (one receiving paclitaxel; one receiving docetaxel). Both women experienced grade 3/4 myalgias and arthralgias that were nonresponsive to acetaminophen and dexamethasone.[42] Neither patient had sensory neuropathy. Based on its efficacy in the treatment of neuropathic and myofascial pain, oral gabapentin (300-2,400 mg/d) was started 24 to 48 hours prior to the taxane and continued for a period of time based on the patient's symptoms during the previous cycle.[42] Gabapentin was reported to significantly reduce myalgias and arthralgias in these patients.
• Glutamine-Glutamine appears to be effective in the prevention of paclitaxel-induced myalgias and arthralgias. Glutamine is a neutral gluconeogenic amino acid involved in whole-body nitrogen metabolism. In animal models, oral glutamine ameliorates neuropathy due to vincristine.[ 43]
Prophylactic glutamine was effective in five patients experiencing moderate to severe myalgias and arthralgias after their first paclitaxel dose (175-200 mg/m2 over 1-3 hours).[31] Glutamine, 10 g orally three times daily, beginning 24 hours after paclitaxel, prevented myalgias and arthralgias in all five patients. The paclitaxel dose was not altered, and two of the five patients continued paclitaxel after these two doses without recurrence of myalgias and arthralgias.
Glutamine has also been evaluated for the prevention of paclitaxelrelated peripheral neuropathy.[44] In addition, there are ongoing trials of the efficacy of glutamine in decreasing the neuromuscular toxicity of paclitaxel (Joseph Bubalo, PharmD, personal communication).
Myalgias/Arthralgias Associated With Other Taxanes
Myalgias and arthralgias have been reported after administration of some, but not all, investigational taxanes.[ 45] Myalgias and arthralgias are infrequently reported with docetaxel, either as monotherapy[41,42] or in combinations.[46] The lower incidence of myalgias and arthralgias with docetaxel may be due to the longer duration of associated dexamethasone administration (ie, six doses over 3 days, compared with one or two doses immediately preceding paclitaxel).[1,9,38] However, a sometimes disabling flu-like syndrome may be seen when corticosteroids are discontinued, possibly representing delayed myalgias and arthralgias caused by docetaxel. Nevertheless, the overall incidence of sensory neuropathy appears to be lower with docetaxel than that with paclitaxel administration.[47]
Conclusions
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Acetaminophen
Calcitonin, intranasal (Miacalcin)
Carboplatin (Paraplatin)
Cisplatin
Dexamethasone
Docetaxel (Taxotere)
Fexofenadine (Allegra)
Gabapentin (Neurontin)
Glutamine
Granulocyte colony-stimulating
factor (G-CSF, Neupogen)
Ibuprofen
Nortriptyline
Paclitaxel
Prednisone
Propoxyphene
Terfenadine (Seldane)
Vincristine
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Myalgias and arthralgias occur frequently among patients receiving paclitaxel. Paclitaxel-induced myalgias and arthralgias are related to individual doses, although the association with total dose and infusion duration is less clear. Identification of risk factors could distinguish a group of patients at greater risk for myalgias and arthralgias and minimize these complications through preventive therapies. Uniform assessment for myalgias and arthralgias within clinical trials would assist in comparing data from different centers and allow a more exact characterization of both the incidence and severity of these adverse effects.
Optimal treatment and prevention of myalgias and arthralgias associated with paclitaxel is unclear, partially due to the small number of patients who have been treated with any one therapy. The most frequently documented pharmacologic intervention is the use of NSAIDs, which have proven effective in many patients, but no clear choice exists for patients who fail to respond to NSAIDs. Given the relatively small number of associated adverse effects, there is considerable enthusiasm for gabapentin, glutamine, and, potentially, antihistamines.
Anecdotally, the use of NSAIDs and corticosteroids in patients experiencing paclitaxel-induced myalgias and arthralgias at our institution has been disappointing. In these patients, the administration of nortriptyline (25 mg orally at bedtime) leads to partial relief, and complete relief only with the addition of fexofenadine. In hopes of minimizing the impact of myalgias and arthralgias, we are starting to use oral glutamine (10 g three times daily for 4 days) in patients refractory to NSAIDs. Patients with peripheral neuropathy are similarly treated with glutamine, on the same dosage schedule.
Ongoing evaluation of this treatment algorithm as well as prospective randomized clinical trials will hopefully improve management of paclitaxel-induced myalgias and arthralgias. The numbers of patients treated with corticosteroids, nortriptyline, and/or fexofenadine, however, have been limited, and it is difficult to draw any conclusions from these observations.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Natale RB: A phase I/II trial of combinationpaclitaxel and carboplatin in advanced ormetastatic non-small cell lung cancer: Preliminaryresults of an ongoing study. Semin Oncol22(6 suppl 15):34-37, 1995.
2.
Vermorken JB, ten Bokkel Huinink WW,Mandjes IA, et al: High-dose paclitaxel withgranulocyte colony-stimulating factor in patientswith advanced breast cancer refractory toanthracycline therapy: A European Cancer Centertrial. Semin Oncol 22(4 suppl 8):16-22, 1995.
3.
Hainsworth JD, Urba WJ, Hon JK, et al:One-hour paclitaxel plus carboplatin in the treatmentof advanced non-small cell lung cancer:Results of a multicentre, phase II trial. Eur JCancer 34:654-658, 1998.
4.
Rowinsky EK, Eisenhauer EA, ChaudhryV, et al: Clinical toxicities encountered withpaclitaxel (Taxol). Semin Oncol 20(4 suppl 3):1-15, 1993.
5.
McGuire WP, Rowinsky EK, RosensheinNB, et al: Taxol: A unique antineoplastic agentwith significant activity in advanced ovarianepithelial neoplasms. Ann Intern Med 111:273-279, 1989.
6.
Smith RE, Brown AM, Mamounas EP, etal: Randomized trial of 3-hour versus 24-hourinfusion of high-dose paclitaxel in patients withmetastatic or locally advanced breast cancer:National Surgical Adjuvant Breast and BowelProject Protocol B-26. J Clin Oncol 17:3403-3411, 1999
7.
Mross K, Hauns B, Haring B, et al: Clinicalphase I study with one-hour paclitaxel infusion.Ann Oncol 9:569-572, 1998.
8.
Holmes FA, Walters RS, Theriault RL, etal: Phase II trial of Taxol, an active drug in thetreatment of metastatic breast cancer. J NatlCancer Inst 83:1797-1805, 1991.
9.
Schiller JH, Storer B, Tutsch K, et al:Phase I trial of 3-hour infusion of paclitaxelwith or without granulocyte colony-stimulatingfactor in patients l 12:241-248, 1994.
10.
Seidman AD, Tiersten A, Hudis C, et al:Phase II trial of paclitaxel by 3-hour infusion asinitial and salvage chemotherapy for metastaticbreast cancer. J Clin Oncol 13:2575-2581,1995.
11.
Eisenhauer EA, ten Bokkel Huinink WW,Swenerton KD, et al: European-Canadian randomizedtrial of paclitaxel in relapsed ovariancancer: High-dose versus low-dose and longversus short infusion. J Clin Oncol 12:2654-2666, 1994.
12.
Nabholtz JM, Gelmon K, Bontenbal M,et al: Multicenter, randomized comparativestudy of two doses of paclitaxel in patients withmetastatic breast cancer. J Clin Oncol 14:1858-1867, 1996.
13.
Seewaldt VL, Greer BE, Cain JM, et al:Paclitaxel (Taxol) treatment for refractory ovariancancer: Phase II clinical trial. Am J ObstetGynecol 170:1666-1670, 1994.
14.
Rowinsky EK, Cazenave LA, DonehowerRC: Taxol: A novel investigational antimicrotubuleagent. J Natl Cancer Inst82:1247-1259, 1990.
15.
Greco FA, Hainsworth JD: Paclitaxel(Taxol): Phase I/II trial comparing 1-hour infusionschedules. Semin Oncol 21(5 suppl 8):3-8,1994.
16.
Millward MJ, Bishop JF, Friedlander M,et al: Phase II trial of a 3-hour infusion ofpaclitaxel in previously untreated patients withadvanced non-small-cell lung cancer. J ClinOncol 14:142-148, 1996.
17.
Criteria for the evaluation of direct effectsof solid cancer chemotherapy. J Jpn SocCancer Ther 28:101-130, 1993.
18.
Apfel SC: Managing the neurotoxicityof paclitaxel (Taxol) and docetaxel (Taxotere)with neurotrophic factors. Cancer Invest18:564-573, 2000.
19.
Roytta M, Raine CS: Taxol-induced neuropathy:Chronic effects of local injection. JNeurocytol 15:483-496, 1986.
20.
Roytta M, Horwitz SB, Raine CS: Taxolinducedneuropathy: short-term effects of localinjection. J Neurocytol 13:685-701, 1984.
21.
Roytta M, Raine CS: Taxol-induced neuropathy:Further ultrastructural studies of nervefibre changes in situ. J Neurocytol 14:157-175,1985.
22.
Masurovsky EB, Peterson ER, Crain SM,et al: Microtubule arrays in Taxol-treated mousedorsal root ganglion-spinal cord cultures. BrainRes 217:392-398, 1981.
23.
Vuorinen V, Roytta M, Raine CS: Theacute effects of Taxol upon regenerating axonsafter nerve crush. Acta Neuropathol (Berl)76:26-34, 1988.
24.
Vuorinen V, Roytta M, Raine CS: Theacute response of Schwann cells to Taxol afternerve crush. Acta Neuropathol (Berl) 76:17-25, 1988.
25.
Kunitoh H, Saijo N, Furuse K, et al:Neuromuscular toxicities of paclitaxel 210 mgm(-2) by 3-hour infusion. Br J Cancer 77:1686-1688, 1998.
26.
Rowinsky EK, Gilbert MR, McGuire WP,et al: Sequences of Taxol and cisplatin: A phaseI and pharmacologic study. J Clin Oncol9:1692-1703, 1991.
27.
Bonomi P, Kim K, Fairclough D, et al:Comparison of survival and quality of life inadvanced non-small-cell lung cancer patientstreated with two dose levels of paclitaxel combinedwith cisplatin versus etoposide with cisplatin:Results of an Eastern CooperativeOncology Group trial. J Clin Oncol 18:623-31,2000.
28.
Onetto N, Canetta R, Winograd B, et al:Overview of Taxol safety. J Natl Cancer InstMonogr 15:131-139, 1993.
29.
Kosmidis P, Mylonakis N, Skarlos D, etal: Paclitaxel (175 mg/m2) plus carboplatin (6AUC) versus paclitaxel (225 mg/m2) plus carboplatin(6 AUC) in advanced non-small-celllung cancer (NSCLC): A multicenter randomizedtrial. Hellenic Cooperative OncologyGroup (HeCOG). Ann Oncol 11:799-805, 2000.
30.
Postma TJ, Vermorken JB, Liefting AJ,et al: Paclitaxel-induced neuropathy. Ann Oncol6:489-494, 1995.
31.
Savarese D, Boucher J, Corey B:Glutamine treatment of paclitaxel-induced myalgiasand arthralgias. J Clin Oncol 16:3918-3919, 1998.
32.
Villalona-Calero MA, Weiss GR, BurrisHA, et al: Phase I and pharmacokinetic studyof the oral fluoropyrimidine capecitabine incombination with paclitaxel in patients withadvanced solid malignancies. J Clin Oncol17:1915-1925, 1999.
33.
Gordon AN, Stringer CA, Matthews CM,et al: Phase I dose escalation of paclitaxel inpatients with advanced ovarian cancer receivingcisplatin: Rapid development of neurotoxicityis dose-limiting. J Clin Oncol15:1965-1973, 1997.
34.
Choy H, Akerley W, Safran H, et al:Phase I trial of outpatient weekly paclitaxeland concurrent radiation therapy for advancednon-small-cell lung cancer. J Clin Oncol.12:2682-2686, 1994.
35.
Johnson DH, Paul DM, Hande KR, et al:Paclitaxel plus carboplatin in advanced non-small-cell lung cancer: A phase II trial. J ClinOncol 14:2054-2060, 1996.
36.
Sarris AH, Younes A, McLaughlin P, etal: Cyclosporin A does not reverse clinical resistanceto paclitaxel in patients with relapsednon-Hodgkin’s lymphoma. J Clin Oncol14:233-239, 1996.
37.
Paul DM, Garrett AM, Meshad M, et al:Paclitaxel and 5-fluorouracil in metastatic breastcancer: The US experience. Semin Oncol 23(1suppl 1):48-52, 1996.
38.
Markman M, Kennedy A, Webster K, etal: Use of low-dose oral prednisone to preventpaclitaxel-induced arthralgias and myalgias.Gynecol Oncol 72:100-101, 1999.
39.
Thomas P, Castelnau O, Paillotin D, etal: Phase II trial of paclitaxel and carboplatin inmetastatic small-cell lung cancer: A GroupeFrancais de Pneumo-Cancerologie study. J ClinOncol 19:1320-1325, 2001.
40.
Martoni A, Zamagni C, Gheka A, et al:Antihistamines in the treatment of Taxol-inducedparoxystic pain syndrome. J Natl CancerInst 85:676, 1993.
41.
Hall RK, Marshall M: Intranasal calcitoninameliorates the arthralgias/myalgias associatedwith taxane chemotherapy (abstract2453). Proc Am Soc Clin Oncol 19:623a, 2000.
42.
van Deventer H, Bernard S: Use of gabapentinto treat taxane-induced myalgias. J ClinOncol 17:434-435, 1999.
43.
Boyle FM, Wheeler HR, Shenfield GM:Glutamate ameliorates experimental vincristineneuropathy. J Pharmacol Exp Ther 279:410-415, 1996.
44.
Vahdat L, Papadopoulos K, Lange D, etal: Reduction of paclitaxel-induced peripheralneuropathy with glutamine. Clin Cancer Res7(5):1192-1197, 2001.
45.
Hidalgo M, Aylesworth C, HammondLA, et al: Phase I and pharmacokinetic study ofBMS-184476, a taxane with greater potencyand solubility than paclitaxel. J Clin Oncol19:2493-2503, 2001.
46.
Cardoso F, Ferreira Filho AF, Crown J,et al: Doxorubicin followed by docetaxel versusdocetaxel followed by doxorubicin in theadjuvant treatment of node positive breast cancer:Results of a feasibility study. AnticancerRes 21(1B):789-795, 2001.
47.
Vasey PA, Atkinson R, Coleman R, etal: Docetaxel-carboplatin as first line chemotherapyfor epithelial ovarian cancer. Br J Cancer84:170-178, 2001.