Nausea and Vomiting: Managing Side Effects From PARP Inhibitors
Here, two oncology pharmacists discuss the side effects of PARP inhibitor treatment, and how to prevent and treat nausea and vomiting in this patient population.
Oncology (Williston Park). 33(2):58-61.

Christine C. Davis, PharmD, BCOP

Sarah Caulfield, PharmD, BCOP

Figure. Illustration of Olaparib Mechanism Specifically in BRCA-Deficient Cells Compared With Normal Cells
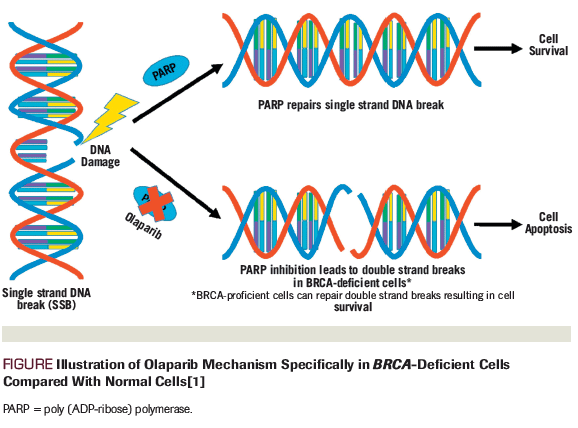
Table 1. Indications of FDA-Approved PARP Inhibitors
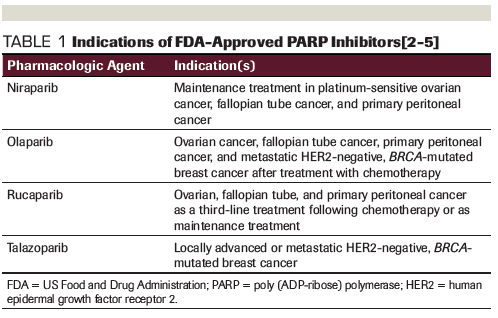
Table 2. National Comprehensive Cancer Network Categorization of Emetic Potential for Intravenous Agents
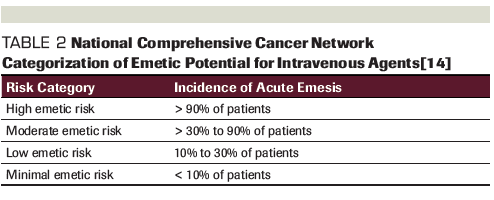
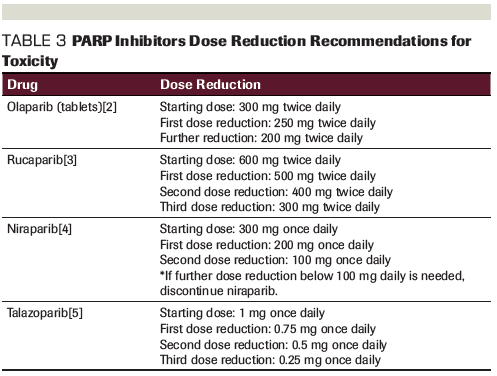
Table 3. PARP Inhibitors Dose Reduction Recommendations for Toxicity
Introduction
Poly (ADP-ribose) polymerase (PARP) inhibitors are a class of oral anticancer medications that have the most evidence for use in patients with inherited germline mutations in BRCA1/2 tumor suppressor genes. By inhibiting PARP, these agents form PARP-DNA complexes, resulting in DNA damage, apoptosis, and cancer cell death (Figure).[1]
The first US Food and Drug Administration (FDA) approval of a PARP inhibitor, olaparib, occurred in 2014; there are now four FDA-approved PARP inhibitors: olaparib, rucaparib, niraparib, and talazoparib (Table 1). Olaparib has four indications: three in ovarian, fallopian tube, and primary peritoneal cancer, and one in metastatic human epidermal growth factor receptor 2 (HER2)-negative, BRCA-mutated breast cancer after treatment with chemotherapy.[2] Rucaparib is indicated in ovarian, fallopian tube, and primary peritoneal cancer as a third-line treatment following chemotherapy or as maintenance treatment.[3] Niraparib is indicated only for maintenance treatment in platinum-sensitive ovarian, fallopian tube, and primary peritoneal cancer.[4] Talazoparib has one indication for treatment in locally advanced or metastatic HER2-negative, BRCA-mutated breast cancer.[5]
As this class of medication becomes more commonly prescribed and continues to gain new indications, it is important that providers address the most common adverse effects of these medications, which include anemia, neutropenia, gastrointestinal (GI) toxicity, and fatigue. When prescribing oral agents with known risk of GI toxicity, providers must proactively prescribe medications to prevent these toxicities, such as nausea and vomiting, in order for the patient to remain adherent to the prescribed dose without significant decline in quality of life.[6] We will outline here how to prevent and treat nausea and vomiting among patients being treated with PARP inhibitors.
PARP Inhibitor–Associated GI Toxicity
In the 2014 Kaufman trial, as well as the SOLO-1 and SOLO-2 trials, nausea was the most commonly reported adverse event seen with olaparib treatment (all grades, 62%–77%; grade ≥ 3, 1%–6%), with a lower incidence of vomiting (all grades, 35%–40%; grade ≥ 3, 1%–3%).[7-9] In the ARIEL2 and ARIEL3 trials, nausea was also the most common adverse event in the rucaparib group (all grades, 75%; grade ≥ 3, 4%); vomiting was also less common (all grades, 37%–42%; grade ≥ 3, 2%–4%).[10,11] Similarly, the NOVA trial showed that the most common adverse effect with niraparib was nausea (all grades, 74%; grade ≥ 3, 3%), with vomiting of all grades occurring in 34% of patients (grade ≥ 3, 2%).[12] However, nausea and vomiting were less prevalent with talazoparib in the EMBRACA trial (for nausea: all grades, 49%, grade ≥ 3, < 1%; for vomiting: all grades, 24%, grade ≥ 3, 2%).[13]
As opposed to other toxicities wherein grade 3 and 4 are seen as more clinically significant, grade 1 nausea and vomiting can cause a significant impact on quality of life. The National Comprehensive Cancer Network (NCCN) guidelines for antiemesis categorize the emetogenic potential of oral anticancer agents based on the frequency of vomiting/emesis reported (Table 2). Treatments are considered to have a moderate to high frequency if emesis occurs in ≥ 30% of patients, which places olaparib, niraparib, and rucaparib in this category. However, talazoparib would be considered to have minimal to low emetic risk.[14]
Management of Nausea and Vomiting
When assessing a patient’s risk for chemotherapy-induced nausea and vomiting (CINV), it is important to consider not only the emetic risk of the therapeutic agent, but also to take into account the individual patient’s risk factors. Women, younger patients, patients with lower alcohol intake history, and those with a history of motion sickness are at increased risk of experiencing CINV during treatment.[14,15] For oral cancer agents that fall in the moderate to high emetic risk category (including olaparib, rucaparib, and niraparib), the NCCN recommends that the patient take a serotonin (5-HT3) receptor antagonist, such as ondansetron 8 to 16 mg daily, ideally approximately 30 minutes before a PARP inhibitor dose.[14] All four of the PARP inhibitors currently approved can be taken with or without food; therefore, the patient should be counseled to take the medication after a meal if they experienced nausea taking it on an empty stomach.[2-5] Of note, olaparib and rucaparib are both dosed twice daily and may require the anti-nausea medication to also be taken twice per day.[2,3] Niraparib is taken only once daily and is recommended to be taken at night to help prevent CINV.[4] Compliance to antiemetic therapy should be encouraged when initiating new PARP inhibitor therapy to avoid drug intolerance and potential for unnecessary interruption in therapy. At each clinic visit, nausea and vomiting should be assessed to determine if any adjustments need to be made to the antiemetic regimen.
For patients who experience CINV during treatment, it should first be determined if they are compliant with their antiemetic regimen, refractory to the antiemetic therapy (experiencing CINV with the majority of doses), or experiencing breakthrough nausea with only occasional episodes. If first-line prevention with a 5-HT3 receptor antagonist is not sufficient, the patient should try alternative treatments for CINV. The general recommendation for patients who are refractory to initial therapy is to stop the first-line agent and switch to a drug with a different mechanism. For patients who experience only breakthrough CINV, it is recommended to add one agent from a different drug class to the current regimen.
The NCCN also recommends olanzapine, lorazepam, dronabinol, haloperidol, metoclopramide, scopolamine transdermal patch, prochlorperazine or promethazine, an alternative 5-HT3 receptor antagonist such as dolasetron or granisetron, or a steroid such as dexamethasone for the prevention of CINV. When deciding between these options, one should consider patient- and drug-specific factors. For example, olanzapine should be started with a low dose and it is recommended that it should be taken at night, since it can cause significant drowsiness. Age should be considered when using benzodiazepines such as lorazepam, since they can cause more complications in elderly patients, though they may be helpful if the patient is experiencing anticipatory nausea. Dronabinol may be useful if the patient is also experiencing decreased appetite. The scopolamine patch may help if the patient finds that they are more sensitive to motion sickness while on PARP inhibitor therapy. Granisetron is also available as a patch if the patient is vomiting and unable to keep down oral medications. Dexamethasone should be recommended with caution, since there are notable complications with long-term use of steroids and it may need to be used daily with PARP inhibitor–related nausea. If a patient vomits after a medication dose, it is important to counsel them to not take another dose until the next scheduled dose. For patients who continue to experience CINV after attempted antiemetic optimization and have compromised quality of life, dose reduction may be required (Table 3).
Additional Considerations With PARP Inhibitors
Drug interactions should be taken into account when choosing which PARP inhibitor to prescribe. For example, a dose reduction for olaparib would be required if given with cytochrome P450 3A4 (CYP3A4) inhibitors.[2] Aprepitant or fosaprepitant, netupitant, and fosnetupitant should all be avoided in patients on olaparib treatment, since they have interactions with medications metabolized through CYP3A4. Patients should also be counseled to avoid grapefruit juice and Seville oranges when taking olaparib.[2] For patients on talazoparib, a dose reduction is required when given with P-glycoprotein inhibitors.[5]
Additionally, mild increases (grade 1 or 2) in serum creatinine are possible due to transporter inhibition with olaparib and rucaparib. Continuation of therapy during evaluation is recommended to rule out true renal dysfunction.[6] Mild increases in transaminases (aspartate and alanine transaminases) may be seen within the first 28 days of rucaparib treatment. Providers should note that they do not need to hold the medication unless transaminases exceed 5 times the upper limit of normal (ULN) or more than 3 times the ULN for bilirubin and more than 2 times the ULN for alkaline phosphatase.[6] Lastly, additional GI adverse effects that should be monitored with PARP inhibitor use include constipation, diarrhea, abdominal pain, dyspepsia, dysgeusia, and decreased appetite.[2-5]
Conclusion
Providers should be aware of the potential risk for GI toxicity with PARP inhibitors. Patients initiating olaparib, rucaparib, or niraparib should receive prophylactic antiemetic medication to help prevent nausea and vomiting throughout the treatment course. Patients should be monitored for compliance and efficacy of antiemetic therapy to help preserve quality of life and prevent inappropriate dose reduction. There are several different classes of medication available for use as antiemetic therapy if initial therapy is not sufficient or is contraindicated. Each patient is different and will require continued monitoring and potential alteration in antiemetic therapy to help them remain compliant and maintain a good quality of life.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Dziadkowiec K, GÄ siorowska E, Nowak-Markwitz E, Jankowska A. PARP inhibitors: review of mechanisms of action and BRCA1/2 mutation targeting. Prz Menopauzalny. 2016;15:215-9.
2. Olaparib (Lynparza) prescribing information. https://www.azpicentral.com/lynparza_tb/pi_lynparza_tb.pdf#page=1. Accessed December 30, 2018.
3. Rucaparib (Rubraca) prescribing information. https://clovisoncology.com/media/1094/rubraca-prescribing-info.pdf. Accessed December 30, 2018.
4. Niraparib (Zejula) prescribing information. https://www.zejula.com/prescribing-information. Accessed December 30, 2018.
5. Talazoparib (Talzenna) prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?id=11046. Accessed December 30, 2018.
6. Gunderson CC, Matulonis U, Moore KN. Management of the toxicities of common targeted therapeutics for gynecologic cancers. Gynecol Oncol. 2018;148:591-600.
7. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244-50.
8. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-505.
9. Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-84.
10. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75-87.
11. Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-61.
12. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-64.
13. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753-63.
14. National Comprehensive Cancer Network. Antiemesis. Version 3.2018. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed December 30, 2018.
15. Mustian KM, Devine K, Ryan JL, et al. Treatment of nausea and vomiting during chemotherapy. US Oncol Hematol. 2011;7:91-7.

Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.