NCCN Updates CRC Guidelines
This special “annual highlights” supplement to Oncology News International (ONI)is a compilation of selected news on important advances in the management ofgastrointestinal cancers over the past year, as reported in ONI. Guest Editor, Dr.James L. Abbruzzese, comments on the reports included herein and discussesdevelopments in the clinical management of GI cancers, with a look at the impactof targeted agents with cytotoxic chemotherapy, first-line and adjuvant therapies foradvanced disease, and the role of statins and COX-2 inhibitors in prevention.
HOLLYWOOD, Florida-Advances over the last several yearshave had a clear impact on medianoverall survival in the first-line treatmentof metastatic colorectal cancerand have led to revisions in the NationalComprehensive Cancer Network(NCCN) guidelines, Paul F. Engstrom,MD, of Fox Chase CancerCenter, said at the NCCN 9th AnnualConference. Dr. Engstrom served aschairperson of the NCCN Colon CancerPanel.Median overall survival has increasedfrom about 6 months with notreatment, to published results of 12.6months in 2002 with 5-FU/leucovorin (5-FU/LV) bolus to about 21 monthsrecently with FOLFOX6 (oxaliplatin[Eloxatin]/infusional 5-FU/LV) andFOLFIRI (irinotecan [Camptosar]/infusional5-FU/LV or folinic acid)."We clearly have combinations thatwork," Dr. Engstrom stated, notingthat oncologists have three options forfirst-line therapy that are "probablyall equivalent." The three options areFOLFOX, FOLFIRI, and bevacizumab(Avastin)/5-FU/LV.FOLFOX/FOLFIRIIn introducing the NCCN recommendations,Dr. Engstrom addressedthree questions. First: Should FOLAgentFOX replace 5-FU/LV as first-linetreatment? Answering in the affirmative,he cited the de Gramont study (JClin Oncol 18:2938, 2000), which comparedfirst-line LV/5-FU2 and FOLFOX4regimens.The 420-patient study, poweredonly to show differences in progression-free survival, revealed highly significantadvantages for FOLFOX inprogression-free survival (9.0 monthsvs 6.2 months, P = .0003) and overallresponse rate (51% vs 22%, P = .001),with a favoring trend in median survival(16.2 months vs 14.7 months).Furthermore, he said, in the N9741trial (Goldberg et al: J Clin Oncol 22:23,2004) median time to tumor progressionwas significantly longer with FOLFOX4(8.7 months) than with IFL(irinotecan/bolus 5-FU/LV) (6.9months) or IROX (oxaliplatin/irinotecan)(6.5 months). Median overallsurvival was significantly longer withFOLFOX4 (19.4 vs 17.6 months forIROX and 14.6 months for IFL).The price in toxicities with the oxaliplatin-bearing regimens is increasedneutropenia and paresthesias. Withirinotecan, it is in higher rates of nausea,vomiting, and diarrhea.The Tournigand et al study (J ClinOncol 22:229-237, 2004) comparingfirst-line FOLFIRI and FOLFOX6 revealedcomparable overall survival(20.6 months for FOLFOX6 and 21.5months for FOLFIRI), progressionfreesurvival, and time to progression.Neurotoxicity (34% vs 0%) andneutropenia (44% vs 24%) were morecommon in the FOLFOX6 arm. Nausea(3% vs 13%), vomiting (3% vs10%), stomatitis (1% vs 10%), andalopecia (grade 2, 9% vs 24%) weremore common in the FOLFIRI arm."It looks like a choice between toxicityprofiles," Dr. Engstrom commented.Novel Agents: Cetuximaband BevacizumabThe second question concerned useof the monoclonal antibody cetuximab(Erbitux). The EGFR (epidermalgrowth factor receptor) that cetuximabtargets is found in at least 50% ofpatients with colon cancer, and itsoverexpression is related to worseprognosis and more advanced diseasestage, Dr. Engstrom said.
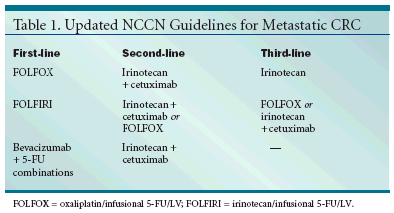
In trials of cetuximab monotherapyvs cetuximab/irinotecan, the combinationproduced significantly higheroverall response rates and longertimes to progression. Appearance ofan acne-like rash in about 30% of patientscorrelates with better response.Dr. Engstrom stated that cetuximabshould be used only in patients whoare positive for EGFR.The last question: How should theantiangiogenesis agent bevacizumabbe integrated into guidelines? A trialin metastatic colorectal cancer (Hurwitzet al: ASCO 2003, abstract 3646)compared IFL, IFL/bevacizumab, and5-FU/LV/bevacizumab. The latter armwas discontinued because of high toxicities.The IFL/bevacizumab combinationproduced significantly longer medianoverall survival (20.3 vs 15.6 months,P = .00003); progression-free survival(10.6 vs 6.24 months, P < .00001);overall response rate (45% vs 35%, P =.0029); and duration of response (10.4vs 7.1 months, P = .0014).Hypertension was significantlymore common with the combination(22.4% vs 8.3%, P < .01), with morepatients requiring dose adjustmentsor addition of new antihypertensivemedications (10.9% vs 2.3%).Because GI perforations were reportedin 1.5% of IFL/bevacizumabpatients and in 0% of IFL/placebo patients,Dr. Engstrom recommendedagainst use of the combination in patientswithin 1 or 2 months of recentsurgery.Dr. Engstrom characterized the second-line options as "very good" (seeTable 1). "The committee feels thatIFL should not be in the armamentarium,"he emphasized, adding thatFOLFIRI is preferred. Where irinotecanis not tolerated, cetuximab alonemay be substituted. Furthermore,5-FU/LV alone or single-agent capecitabine(Xeloda) should be used only inpatients who cannot tolerate a multidrugregimen.Adjuvant TherapyRegarding the question as towhether FOLFOX could replace 5-FU/LV as adjuvant therapy in stage IIIcolorectal cancer, Dr. Engstrom pointedto the MOSAIC trial, which comparedLV/5-FU2 with FOLFOX4 instage II-III colorectal cancer patients.Disease-free survival, which was theprimary endpoint, was reduced 24%at 3-years in the stage III patients withFOLFOX4 (71.8% vs 65.5%) and 18%in the stage II patients (86.6% vs83.9%)."We think that FOLFOX is an optionto replace 5-FU/LV in the adjuvantsetting, but you must considerthe neurotoxicity, which the elderly,especially, may not be able to tolerate,"Dr. Engstrom said.
Targeted Therapy First Strategy Reduces Need for Chemotherapy in Newly Diagnosed LBCL
December 7th 2025Lenalidomide, tafasitamab, rituximab, and acalabrutinib alone may allow 57% of patients with newly diagnosed LBCL to receive less than the standard number of chemotherapy cycles without compromising curative potential.