Neoadjuvant Chemotherapy for Resectable Non–Small-Cell Lung Cancer
Lung cancer is the most common cancer diagnosed in men and women in the United States, and is the leading cause of cancer death.Over 160,000 individuals died as a result of lung cancer in 2008.[1] This number amounted to more than the number of deaths from colon, breast, and prostate cancers combined. The majority of lung cancer cases are non–small-cell lung cancer (NSCLC), and the poor outcomes are attributed to the high rate of metastases associated with this disease.
ABSTRACT: Lung cancer generally has an unfavorable prognosis. For those with resectable disease, the use of neoadjuvant chemotherapy has the potential to reduce tumor volume, address micrometastatic disease early, and improve outcomes. Randomized trials comparing neoadjuvant platinum-based regimens with surgery alone were able to demonstrate the feasibility and safety of this modality. These trials supported evidence found in phase II trials that utilized third-generation chemotherapies. Still, limitations to these studies exist, such as the inclusion of various disease stages in one study, inter- and intratrial variability of the chemotherapy regimens used, and lack of phase III data comparing neoadjuvant to adjuvant chemotherapy. These heterogeneous factors make it difficult to offer firm recommendations about neoadjuvant chemotherapy. Other matters of contention include the role of postoperative radiation and the concern for increased postoperative complications, especially when a right pneumonectomy is being considered after neoadjuvant chemotherapy. To clarify these issues, well-structured phase III trials comparing adjuvant to neoadjuvant chemotherapy are needed.
Lung cancer is the most common cancer diagnosed in men and women in the United States, and is the leading cause of cancer death.
TABLE 1
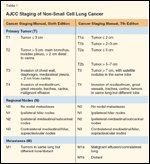
AJCC Staging of Non-Small Cell Lung Cancer
Over 160,000 individuals died as a result of lung cancer in 2008.[1] This number amounted to more than the number of deaths from colon, breast, and prostate cancers combined. The majority of lung cancer cases are non–small-cell lung cancer (NSCLC), and the poor outcomes are attributed to the high rate of metastases associated with this disease. Most patients diagnosed with NSCLC present with stage IIIB or IV (Table 1). These late stages are unresectable and have grave prognoses (1%–5% 5-year survival).[2,3] Even those with early-stage (stage I–IIIA), resectable disease are likely to succumb to recurrent disease. The rates of recurrence and death at 5 years for patients with stages IB, II, and IIIA NSCLC are high.[4-6]
To reduce the rate of distant metastases, adjuvant platinum-based chemotherapy is now considered the standard of care for patients with stages IIA to IIIA NSCLC and may be considered an option for select patients with stage IB disease. This article will review the neoadjuvant chemotherapy data and explore what role, if any, neoadjuvant chemotherapy plays in patients with resectable NSCLC.
Adjuvant Chemotherapy
Prior to discussing neoadjuvant chemotherapy, a brief review of adjuvant NSCLC chemotherapy is needed. Three large trials and two meta-analyses have demonstrated that platinum-based chemotherapy can result in a survival benefit when given in the adjuvant setting. Before these studies were published, adjuvant chemotherapy was not considered a standard therapy. In 1995, a meta-analysis of 52 randomized trials evaluating cytotoxic chemotherapy in patients with NSCLC was reported. Of these trials, 14 were randomized, pooling together over 4,000 patients. This subset analysis demonstrated that platinum-based regimens (8 studies) vs non–platinum-based regimens (6 studies) did improve survival following surgical resection.[7] Survival was improved by 4% at 2 years, with a 13% reduction in death.
Further platinum-based trials were then developed based on this promising information, and these findings were confirmed in another meta-analysis.[8] The modern trials included the International Adjuvant Lung Trial (IALT), Adjuvant Navelbine International Trialist Association (ANITA), and the National Cancer Institute of Canada Clinical Trials Group JBR.10. The IALT randomized 1,867 participants with stage I to III NSCLC to adjuvant cisplatin-based chemotherapy vs observation and demonstrated an improved overall survival (44.5% vs 40.4%, P < .03) and disease-free survival (39.4% vs 34.3%, P < .003) in the treatment arm at 5 years.[4] The ANITA trial randomized 407 vs 433 patients with stage IB to IIIA NSCLC to cisplatin plus vinorelbine chemotherapy vs observation following surgical resection. At the 7-year follow-up, the ANITA trial demonstrated that overall survival was improved by 8.4% (P = .017).[9] In the JBR.10 trial, cisplatin plus vinorelbine was once again the chemotherapy of choice; this trial randomized 482 patients with stage IB or II NSCLC to adjuvant chemotherapy vs observation. The 5-year survival was 69% vs 54% (P = .03).[6] Results from all three trials changed the outlook and treatment algorithms for patients with early-stage NSCLC.
Both the American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines recommend the use of adjuvant chemotherapy for patients with resectable stage IIA to IIIA NSCLC.
Neoadjuvant Chemotherapy
The role of neoadjuvant chemotherapy has also been explored in patients with early-stage NSCLC. The rationale for further investigations was the hope of decreasing micrometastases at distant sites and tumor burden preoperatively to increase resectability rates and overall survival.
TABLE 2

Randomized Trials of Induction Chemotherapy Compared With Surgery Alone in NSCLC
In the 1990s, two small randomized trials sparked further interest in neoadjuvant chemotherapy in patients with resectable NSCLC because they demonstrated a survival benefit to platinum-based therapy (Table 2).[10,11] These trials had their limitations, which included relatively small patient numbers, unexpectedly poor outcome in the control arm, and inhomogeneous study populations.
In 2006, a meta-analysis evaluating seven neoadjuvant randomized trials involving 988 patients demonstrated an overall survival improvement of 20% in the treatment group compared with 14% (P = .02) in the observation group.[12] Three of the largest studies included in this meta-analysis were responsible for most of the observed affect. The Depierre et al study randomized 355 patients with stage IB to IIIA to two cycles of preoperative cisplatin, ifosfamide, and mitomycin compared with primary surgery.[13] Patients who responded to chemotherapy (64%) received an additional two cycles postoperatively. Overall survival for patients in the treatment arm was increased compared with those in the nontreatment arm (8.6% vs 3.8% at 1 year). This study also provided evidence regarding the safety of preoperative chemotherapy. No excess deaths were found in the preoperative chemotherapy arm compared with the primary surgical arm (6.7% vs 4.5%, P = .38).
Gilligan et al developed a randomized trial that compared neoadjuvant cisplatin-based chemotherapy to surgery alone. This study, which spanned four countries and randomized 588 participants with resectable stage I to III disease, failed to demonstrate a statistically significant improvement in overall survival at 5 years (44% vs 45%, hazard ratio = 1.02). Although 31% of the patients in the chemotherapy arm were downstaged, there was no change in the planned surgery.[14] The investigators found no increase in surgical morbidity in the chemotherapy group. Factors that may have contributed to the negative results included the fact that six different chemotherapy regimens were allowed in this study and the majority of study participants had stage I disease (61%), thus making it more difficult for the study to reach statistical significance.
TABLE 3

Phase II Neoadjuvant Trials in Non-Small-Cell Lung Cancer
At the 2007 annual meeting of the American Society of Clinical Oncology (ASCO), the results of the Southwest Oncology Group (SWOG) 9900 trial were updated. The trial randomized 388 participants to surgery alone vs three cycles of preoperative carboplatin and paclitaxel. A statistically nonsignificant trend toward improved 5-year survival was observed in the experimental arm (43% vs 50%, hazard ratio = 0.81).[15] Phase II studies evaluating cisplatin/vindesine (Eldisine)/ifosfamide,[16] cisplatin/vinblastine,[17] cisplatin/docetaxel (Taxotere),[18] and cisplatin/gemcitabine (Gemzar)[19] combinations have further demonstrated a role for third-generation drugs in the neoadjuvant setting (Table 3). These studies suggest a role for induction chemotherapy for patients with stage II or IIIA NSCLC who are operable candidates.
Limitations
Perhaps the most important limitation of neoadjuvant clinical trials is the inability to properly stage patients prior to surgical resection; ie, all staging is by definition clinical staging (even if mediastinoscopic lymph node sampling is performed). Inaccurate staging can lead to improper treatments. Positron-emission tomography/computed tomography (PET/CT) and mediastinoscopy should be considered in patients who are potentially resectable, to improve the pre–induction chemotherapy staging.
The published trials have used a variety of different chemotherapy regimens including different platinum analogs (cisplatin or carboplatin). Further studies are needed to determine the optimal chemotherapy regimen and to clarify the role of carboplatin in the curative setting. Many studies have included a mixture of stages, which adds to the difficulty of interpreting the data. Future directions for clinical trials should give consideration to dividing trials into stage IB/II vs IIIA, as outcomes vary between these two groups. This should alleviate some of the difficulties in the analysis and interpretation of the clinical data and its subsequent application to the clinical decision-making process.
Risks
Some authors have raised the concern that preoperative chemotherapy may increase the risk of postoperative complications. In 2001, Roberts et al found that neoadjuvant chemotherapy increased the rate of perioperative morbidity.
Matched for age, comorbidities, and pulmonary function, 34 patients who underwent induction chemotherapy were compared with 67 patients who underwent primary surgery. The investigators found an increased rate of life-threatening complications in the neoadjuvant chemotherapy group, including pneumonia, emergency surgery, transfer to the intensive care unit, or intubation (6.0% vs 26.5%, P = .0036), and major complications defined as prolonged hospital stay (19.4% vs 47.1%, P = .0037), reintubation (3.0% vs 17.6%, P = .0093), and tracheostomy (0% vs 11.8%, P = .0042).[20]
One major limitation of this study is the lack of matching for stage between the two groups. Patients in the induction chemotherapy arm had an overall higher stage (2.52 vs 1.55, P < .001). Additionally, findings from this analysis were based on data from a single surgeon at a single institution. Because of these factors, this study should not be used to dictate standard-of-care recommendations. In another retrospective analysis of 106 patients, Venuta et al showed that lobectomy can be safely performed after induction chemotherapy by seasoned surgeons.[21]
TABLE 4

Morbidity and Mortality Associated With Neoadjuvant Chemotherapy in Non–Small-Cell Lung Cancer
Prospective studies have also been done to better assess perioperative risks associated with neoadjuvant chemotherapy. These studies include the larger randomized studies already mentioned (Table 4), which did not demonstrate excessive unexpected morbidity. These studies demonstrated the feasibility and safety of neoadjuvant chemotherapy when used in properly selected patient populations. A criticism has been that these studies pooled all types of pulmonary resections together. When the risks associated with pneumonectomy after induction chemotherapy were assessed, studies supporting and dismissing the safety of neoadjuvant chemotherapy can be found (Table 4).
Mansour et al retrospectively evaluated 306 patients who underwent pneumonectomy from January 1999 to July 2005 and found no significant increase in morbidity or mortality after neoadjuvant chemotherapy.[22] This result is based on a single-site, single-surgeon analysis. Martin et al performed a retrospective analysis of the Memorial Sloan-Kettering Cancer Center thoracic surgical database from 1993 to 1999 and found 479 patients who received induction chemotherapy. This group demonstrated an excess mortality (23.9%) in those who underwent a right pneumonectomy.[23]
In summary, the risks of surgical morbidity and mortality associated with neoadjuvant chemotherapy remain controversial. Although some studies have reported an increased incidence of complications, others have not. Due to the potential excess mortality, a right pneumonectomy after neoadjuvant chemotherapy should only be considered in selected patients and should only be performed by an experienced thoracic surgeon.
Role of Postoperative Radiation Therapy
Phase II studies evaluating the role of induction chemotherapy vary with respect to use of postoperative radiotherapy (PORT). Given concerns over efficacy and safety, this is another controversial area in the treatment of patients with resectable NSCLC. The issue is highlighted by data from 2,128 patients with stage I to III (N2) disease, presented by the PORT Meta-analysis Trialist Group.[24] Overall, PORT was found to be more harmful to patients with resectable lung cancer. A subset analysis suggested that the majority of the detrimental effects occurred in those with stage I/II disease and not in those with N2 disease. Still, this study contained many flaws, such as the inclusion of unpublished data, obsolete radiation techniques now known to be inferior, and inapt staging techniques. More recently, PORT has been evaluated in over 7,400 patients with stage II or III NSCLC, and a benefit was found in those with N2 disease.[25] No benefit was found in those with N0 or N1 disease.
Third-Generation Triplet Combinations
To improve the survival and response rates to preoperative chemotherapy, investigators have explored platinum-based triplet combinations. For example, in a phase II study, 49 patients with stage IIIA (N2) NSCLC received preoperative chemotherapy with cisplatin, paclitaxel, and gemcitabine followed by surgery. Results revealed that 55% of patients underwent complete surgical resection, 73.5% had a reduction in tumor burden, and the 1-year overall survival rate was 85%.[26] Toxicities were associated with the triplet combination, including grade 3/4 neutropenia in 32.7% of patients and thrombocytopenia in 12%.
In another study (a South African phase II trial), the triplet combination of paclitaxel, carboplatin, and gemcitabine was evaluated in patients with operable NSCLC. In the 44 patients with stage IB–IIIA disease, 76.2% had an objective response to therapy, 81% had a complete resection, and the 1-year survival rate was 86%.[27] Toxicities associated with this regimen mainly included grade 3/4 neutropenia (38.6%) and grade 3 thrombocytopenia (25%).
The Spanish Lung Cancer Group trial demonstrated the efficacy of three cycles of cisplatin, gemcitabine, and docetaxel chemotherapy followed by surgery in patients with stage IIIA and IIIB (T4, N0/1) disease, with an overall response rate of 68.9% and 3- and 5-year survival rates of 60.1% and 41.4%, respectively.[28] The incidence of grade 3/4 neutropenia and thrombocytopenia was 62.5% and 25.7%, respectively. These studies showed the feasibility of delivering neoadjuvant platinum-based triplet chemotherapy to operable candidates with NSCLC. The use of these regimens translated into a higher response rate (70% vs 60%) compared with historical controls of platinum doublets. The toxicities seen in these studies were primarily hematologic, and the rates of hematologic toxicities do exceed those seen previously with platinum doublets.[29]
To definitively determine whether triplet combinations improve outcomes compared to doublet regimens, a phase III trial is needed. Participants in this study would need to be followed closely. Before such an investigation is started, however, thought must be given to the question of whether it is truly warranted, as survival with the platinum triplets was not superior to the platinum doublets while the toxicities are increased. Based on the current data, the authors of this article do not believe that three-chemotherapy combinations offer significant advantages that would justify the added risks associated with these regimens.
TABLE 5

Randomized Trials of Neoadjuvant Therapy Followed by Surgery Compared With Definitive Chemoradiotherapy for Stage IIIA Non–Small-Cell Lung Cancer
N2 Disease
Although surgery offers the best chance for survival to patients with limited-station N2 disease, not all patients with N2 disease are appropriate candidates for surgical resection. Neoadjuvant systemic chemotherapy may play a role in the management of this disease to help facilitate local surgical control. Many patients may have technically resectable disease but may not be curatively resectable. That is, they will have microscopic or macroscopic disease following their surgical procedure, solidifying their chances for relapse. For these patients, consideration must be given to concurrent chemoradiation for definitive treatment (Table 5). Although phase II studies did suggest a benefit to neoadjuvant concurrent chemoradiation therapy followed by surgery,[30-32] randomized trials found no additional benefit to surgery following bimodality induction therapy.[33-37]
Adjuvant vs Neoadjuvant Chemotherapy
Many studies have called for a randomized trial to evaluate and compare adjuvant vs neoadjuvant chemotherapy in patients with resectable cancer. In the Neoadjuvant/Adjuvant Taxol (paclitaxel) Carboplatin Hope (NATCH) trial from the Spanish Lung group, participants with stage I (> 2 cm), II, and T3, N1 NSCLC were randomized to either neoadjuvant or adjuvant carboplatin/paclitaxel and surgery. The preliminary results from the neoadjuvant arm, which was presented at the 2007 ASCO meeting,[38] found neoadjuvant chemotherapy to be safe and feasible.
Final results of the NATCH trial were presented in August 2009 at the 13th World Congress on Lung Cancer, organized by the International Association for the Study of Lung Cancer (IASLC).[39] Among the 624 participants in the study at the time of presentation, no significant difference in progression-free or overall survival was detected. With preoperative chemotherapy, adjuvant chemotherapy, and surgery alone, the 5-year progression-free survival rates were 38.3%, 36.6%, and 34%, and the 5-year overall survival rates were 46.6%, 45.5%, and 44%, respectively. This study was limited by the fact that a majority of participants had stage IA/IB NSCLC. Moreover, many participants in the adjuvant chemotherapy arm were unable to receive the planned three cycles of chemotherapy due to peri/postoperative morbidity. Both of these factors may have contributed to the difficulty in achieving statistical significance. Nevertheless, subset and exploratory analyses of the trial are currently underway to evaluate prognostic factors as well as prognostic and predictive molecular markers.
The Chinese Society of Lung Cancer has launched the Survival Study of Docetaxel and Carboplatin as Neoadjuvant vs Adjuvant Chemotherapy in Early-Stage NSCLC (NCT00321334). This investigation is set to enroll 410 participants and to be completed in March 2012. The trial may provide long-awaited answers to some key questions.
A systematic review of 31 randomized trials (21 postoperative and 10 preoperative chemotherapy) with over 10,000 subjects presented at ASCO 2008 is available. In this review, no differences in surgical morbidity/mortality, disease-free survival, or overall survival were found between the two groups.[40] Based on these results, it would appear that the timing of chemotherapy administration has little impact on outcomes for patients with operable NSCLC.
Reference Guide
Therapeutic Agents
Mentioned in This Article
Carboplatin
Cisplatin
Docetaxel (Taxotere)
Gemcitabine (Gemzar)
Ifosfamide
Mitomycin
Paclitaxel
Vinblastine
Vindesine (Eldisine)
Vinorelbine
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Future Directions
To determine which chemotherapy regimens are ideal for which patient, molecular analysis is currently being studied in the metastatic and adjuvant setting. Molecular analysis for classification of NSCLC will play a key role as a tool for therapy-related decisions.
Conclusions
Lung cancer carries a poor prognosis. In efforts to improve that prognosis, the role of neoadjuvant chemotherapy has been studied extensively. From the type of surgical procedure performed to the type of chemotherapy used-not only within but also between studies-the data are difficult to analyze because of multiple heterogeneities. Nevertheless, potential advantages of neoadjuvant chemotherapy can be hypothesized. These include a decrease in tumor volume to improve curative surgical resection rates. With systemic administration of chemotherapy, micrometastatic disease is attended to earlier. In addition, there has been some argument for increased compliance with systemic administration of chemotherapy in the neoadjuvant setting. Whether these approaches translate into outcomes superior to those found when platinum-based chemotherapy is used in the adjuvant setting remains unknown.
Evidence about the role of neoadjuvant chemotherapy is inconclusive, as no large randomized trials comparing neoadjuvant chemotherapy to adjuvant chemotherapy in resectable NSCLC have determined any significant differences. The authors of this review believe that each patient needs to be evaluated individually by a multidisciplinary team of physicians.
Neoadjuvant chemotherapy may be beneficial to some patients, especially those who may otherwise have been unresectable. Patients must undergo adequate staging of the mediastinum via modalities such as mediastinoscopy and PET/CT. In addition to staging, the treating physician must pay close attention to the patients’ performance status, age, weight, and comorbidities when making these decisions. Finally, the addition of PORT to the treatment algorithms of patients with stage IIIA disease can be considered.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2008. CA Cancer J Clin 58:71-96, 2008.
2. Mountain CF: Revisions in the International System for Staging Lung Cancer. Chest 111:1710-1717, 1997.
3. Groome PA, Bolejack V, Crowley JJ, et al: The IASLC Lung Cancer Staging Project: Validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2:694-705, 2007.
4. Arriagada R, Bergman B, Dunant A, et al: Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350:351-360, 2004.
5. Kato H, Ichinose Y, Ohta M, et al: A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 350:1713-1721, 2004.
6. Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352:2589-2597, 2005.
7. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 311:899-909, 1995.
8. Pignon JP, Tribodet H, Scagliotti GV, et al: Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 26:3552-3559, 2008.
9. Scagliotti GV, Fossati R, Torri V, et al: Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. J Natl Cancer Inst 95:1453-1461, 2003.
10. Roth JA, Fossella F, Komaki R, et al: A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 86:673-680, 1994.
11. Rosell R, Gomez-Codina J, Camps C, et al: Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: A 7-year assessment of a randomized controlled trial. Lung Cancer 26:7-14, 1999.
12. Burdett S, Stewart LA, Rydzewska L: A systematic review and meta-analysis of the literature: Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. J Thorac Oncol 1:611-621, 2006.
13. Depierre A, Milleron B, Moro-Sibilot D, et al: Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol 20:247-253, 2002.
14. Gilligan D, Nicolson M, Smith I, et al: Preoperative chemotherapy in patients with resectable non-small cell lung cancer: Results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 369:1929-1937, 2007.
15. Pisters K, Vallieres, E, Bunn, PA, et al: S9900: Surgery alone or surgery plus induction (ind) paclitaxel/carboplatin (PC) chemotherapy in early stage non-small cell lung cancer (NSCLC): Follow-up on a phase III trial (abstract 7520). J Clin Oncol 25(18S):389s, 2007.
16. Lorent N, De Leyn P, Lievens Y, et al: Long-term survival of surgically staged IIIA-N2 non-small-cell lung cancer treated with surgical combined modality approach: Analysis of a 7-year prospective experience. Ann Oncol 15:1645-1653, 2004.
17. Sugarbaker DJ, Herndon J, Kohman LJ, et al: Results of cancer and leukemia group B protocol 8935. A multiinstitutional phase II trimodality trial for stage IIIA (N2) non-small-cell lung cancer. Cancer and Leukemia Group B Thoracic Surgery Group. J Thorac Cardiovasc Surg 109:473-485 (incl discussion), 1995.
18. Betticher DC, Hsu Schmitz SF, Totsch M, et al: Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: A multicenter phase II trial. J Clin Oncol 21:1752-1759, 2003.
19. Migliorino MR, De Marinis F, Nelli F, et al: A 3-week schedule of gemcitabine plus cisplatin as induction chemotherapy for stage III non-small cell lung cancer. Lung Cancer 35:319-327, 2002.
20. Roberts JR, Eustis C, Devore R, et al: Induction chemotherapy increases perioperative complications in patients undergoing resection for non-small cell lung cancer. Ann Thorac Surg 72:885-888, 2001.
21. Venuta F, Anile M, Diso D, et al: Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg 31:714-717, 2007.
22. Mansour Z, Kochetkova EA, Ducrocq X, et al: Induction chemotherapy does not increase the operative risk of pneumonectomy! Eur J Cardiothorac Surg 31:181-185, 2007.
23. Martin J, Ginsberg RJ, Abolhoda A, et al: Morbidity and mortality after neoadjuvant therapy for lung cancer: The risks of right pneumonectomy. Ann Thorac Surg 72:1149-1154, 2001.
24. PORT Meta-analysis Trialists Group: Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet 352:257-263, 1998.
25. Lally BE, Zelterman D, Colasanto JM, et al: Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 24:2998-3006, 2006.
26. De Marinis F, Nelli F, Migliorino MR, et al: Gemcitabine, paclitaxel, and cisplatin as induction chemotherapy for patients with biopsy-proven stage IIIA(N2) nonsmall cell lung carcinoma: A phase II multicenter study. Cancer 98:1707-1715, 2003.
27. Abratt RP, Lee JS, Han JY, et al: Phase II trial of gemcitabine-carboplatin-paclitaxel as neoadjuvant chemotherapy for operable non-small cell lung cancer. J Thorac Oncol 1:135-140, 2006.
28. Garrido P, Gonzalez-Larriba JL, Insa A, et al: Long-term survival associated with complete resection after induction chemotherapy in stage IIIA (N2) and IIIB (T4N0-1) non small-cell lung cancer patients: The Spanish Lung Cancer Group Trial 9901. J Clin Oncol 25:4736-4742, 2007.
29. Schiller JH, Harrington D, Belani CP, et al: Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92-98, 2002.
30. Weiden PL, Piantadosi S: Preoperative chemotherapy (cisplatin and fluorouracil) and radiation therapy in stage III non-small-cell lung cancer: a phase II study of the Lung Cancer Study Group. J Natl Cancer Inst 83:266-273, 1991.
31. Weitberg AB, Yashar J, Glicksman AS, et al: Combined modality therapy for stage IIIA non-small cell carcinoma of the lung. Eur J Cancer 29A:511-515, 1993.
32. Albain KS, Rusch VW, Crowley JJ, et al: Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: Mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 13:1880-1892, 1995.
33. Johnstone DW, Byhardt RW, Ettinger D, et al: Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 54:365-369, 2002.
34. Taylor NA, Liao ZX, Cox JD, et al: Equivalent outcome of patients with clinical stage IIIA non-small-cell lung cancer treated with concurrent chemoradiation compared with induction chemotherapy followed by surgical resection. Int J Radiat Oncol Biol Phys 58:204-212, 2004.
35. van Meerbeeck JP, Kramer GW, Van Schil PE, et al: Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 99:442-450, 2007.
36. Albain KS, Swann RS, Rusch VR, et al: Phase III study of concurrent chemotherapy and radiotherapy (CT/RT) vs CT/RT followed by surgical resection for stage IIIA (pN20 non-small cell lung cancer: Outcomes update of North American Intergroup 0139 (RTOG 9309) (abstract 7014). J Clin Oncol 23(16S):624s, 2005.
37. Robinson LA, Ruckdeschel JC, Wagner H Jr, et al: Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132(3 suppl):243S-265S, 2007.
38. Felip E, Rosell R, Massuti B, et al: The NATCH trial: Observations on the neoadjuvant arm (abstract 7578). J Clin Oncol 25(18S):403s, 2007.
39. Felip E, Massuti B, Alonso G, et al: A phase III randomized trial of surgery alone, or preoperative (PREOP) paclitaxel/carboplatin (PC) followed by surgery, or surgery followed by adjuvant (ADJ) PC in early stage non-small cell lung cancer (NSCLC): NATCH follow-up data (abstract PRS.3). 13th World Conference on Lung Cancer, San Francisco, 2009.
40. Lim E, Harris G, Patel I, et al: Preoperative versus postoperative chemotherapy in patients with resectabley non-small cell lung cancer: Systematic review and indirect comparison meta-analysis of randomized trials (abstract 7546). J Clin Oncol 26(15S):408s, 2008.