Neoadjuvant Therapy
This study was conducted to determine whether docetaxel (Taxotere) prior to definitive local treatment improves overall survival when compared to local treatment without prior chemotherapy in patients with radically treatable stage IIIA N2 (T0–3), T3 (N0-1) or IIIB non–small-cell lung cancer. Local treatment was defined at baseline.
This study was conducted to determine whether docetaxel (Taxotere) prior to definitive local treatment improves overall survival when compared to local treatment without prior chemotherapy in patients with radically treatable stage IIIA N2 (T03), T3 (N0-1) or IIIB nonsmall-cell lung cancer. Local treatment was defined at baseline.
A total of 274 patients were randomly assigned to arm Adocetaxel 100 mg/m² (1 h IV) every 3 weeks for three consecutive cycles followed by local treatmentor arm Bimmediate local treatment. Stratification was by extent of disease and World Health Organization (WHO) performance status. Patient characteristics were well balanced in both arms: median age, 62 years (range: 3281 years); male/female ratio, 80%/20%; WHO performance status 01/2, 88%/12%; histologyadenocarcinoma, 20%; squamous cell, 63%; other, 17%.
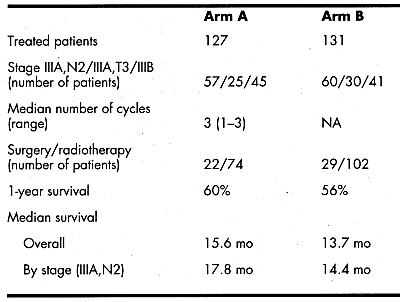
In total, 347 cycles were given. The major toxicity in arm A was grade 4 neutropenia (34% of all cycles). 3.9% of patients experienced febrile neutropenia (1.4% of cycles), and 3.2% experienced grade 3/4 infections. There were two deaths due to infections.
CONCLUSION: Preliminary results show a strong trend in favor of improved survival in the docetaxel-treated patients.
Click here for Dr. Vincent A. Millers commentary on this abstract.