New assay measures HER2 expression more precisely
The HER2-positive breast cancer population appears to be a heterogeneous group with a wide variation in response to trastuzumab (Herceptin). Higher levels of HER2 expression as well as HER2:HER2 dimerization were independently correlated with high response rates and longer time to progression in a study reported at the 2007 San Antonio Breast Cancer Symposium (abstract 2007).
SAN ANTONIO-The HER2-positive breast cancer population appears to be a heterogeneous group with a wide variation in response to trastuzumab (Herceptin). Higher levels of HER2 expression as well as HER2:HER2 dimerization were independently correlated with high response rates and longer time to progression in a study reported at the 2007 San Antonio Breast Cancer Symposium (abstract 2007).
“It appears that not all HER2-positive patients are the same, and not all respond the same to trastuzumab. In a population of patients identified as being HER2 positive, we found differences as great as 50%,” said Weidong Huang, MD, PhD, senior director of clinical research, Monogram Biosciences, South San Francisco, California.
The findings suggest that more precise methods of quantitating HER2 expression or measuring HER2 functionality (dimerization) will allow for improved stratification of metastatic breast cancer patients for trastuzumab treatment.
The study obtained quantitative measurements of total HER2 receptor expression (H2T) and HER2:HER2 dimerization (H22D) in formalin-fixed, paraffin-embedded breast tissue using a novel assay called HERmark that is based on the VeraTag technology platform developed by Monogram Biosciences. VeraTag quantifies proteins and functional protein complexes (see “How it works” box).
“The H2T assay generally correlates with IHC and FISH but is able to measure a continuum of HER2 expression over approximately a 3-log dynamic range,” Dr. Huang said.
The measurements obtained with HERmark were correlated with clinical outcome measures in a cohort of 58 metastatic breast cancer patients treated with trastuzumab-based regimens (90% received chemotherapy plus trastuzumab).
Patients with high H2T and high H22D showed significantly higher objective response rates than those with low H2T and low H22D, respectively, Dr. Huang reported.
While current testing methods identified all these patients as being appropriate for trastuzumab, the assay was able to distinguish separate subpopulations of patients with different clinical outcomes.
Objective response rates were 59.3% for patients with H2T greater than or equal to the median vs 17.9% for patients with H2T below the median (P = .002), including complete responses in 29.6% vs 7.1%, and partial responses in 29.6% vs 10.7%, respectively. Progressive disease was seen in only 14.8% of patients with high expression, compared with 46.4% for those with H2T less than the median, he reported.
Similarly, for H22D greater than or equal to the median, objective responses were seen in 55.6% vs 21.4% (P = .01), complete responses in 29.6% vs 7.1%, partial responses in 25.9% vs 14.3%, and progressive disease in 22.2% vs 39.3%, respectively.
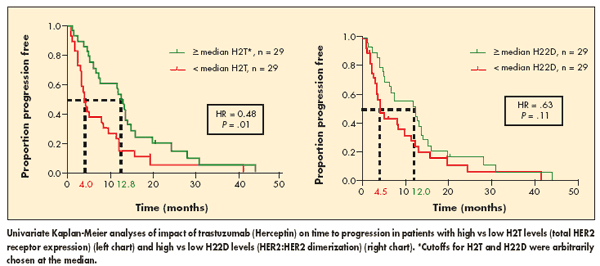
The differences in response rates were reflected in significantly longer time to progression in patients with high H2T, as compared to those with low H2T (see Figure): 12.8 months vs 4.0 months, for a 52% reduction in risk (P = .01). A similar trend was observed for H22D: 12.0 months vs 4.5 months, for a 37% reduction in risk, but this did not reach statistical significance (P = .11).
“The high H2T and H22D groups showed a trend toward better overall survival as well, but this did not reach statistical significance. Further validation of H2T and H22D using overall survival as an endpoint may require larger datasets,” Dr. Huang said.
In a multivariate Cox proportional analysis of eight factors and their relationship to time to progression, H2T and H22D were independent and highly significant correlates (P < .001).
Whether such information could potentially mean that some HER2-positive patients will not be candidates for trastuzumab is “hard to say,” he said, “because there was no control group that did not receive trastuzumab in this study. But this information could eventually help us predict whether trastuzumab will be sufficient, or whether we should put a particular patient on a clinical trial, perhaps with a multi-targeted agent such as lapatinib [Tykerb],” Dr. Huang said.
Monogram Biosciences has initiated a study with a cancer cooperative group in which the HERmark assay will be performed on tissue from up to 1,600 patients treated with trastuzumab in the adjuvant therapy setting.
Concept is important
“The concept behind this technology is very important,” C. Kent Osborne, MD, told ONI in an interview at SABCS. Dr. Osborne is director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Cancer Center at Baylor College of Medicine, Houston.
“We now know that HER2 does not work by itself,” he said. “In fact, in order to activate its pathways, it has to partner with another member of the HER family [heterodimerization]. When HER2 is present in high concentrations, it can also be activated by partnering with itself [homodimerization]. The pathway can be activated by a variety of homo- and heterodimer pairs.”
Trastuzumab blocks only a portion of the HER network, as does lapatinib. Should it become possible to determine which dimer pair was activating the pathway in a given patient’s tumor, clinicians could select a specific agent that would target the relevant pathway, he said.
Incomplete blockade of the pathway allows for an escape route, whereas more complete signal blockade affecting all dimer pairs, using combinations of agents, has been shown to have a powerful effect in preclinical models in Dr. Osborne’s laboratory. Such cocktails have completely eradicated some xenograft models of human breast cancer, he said.
‘We cured the mouse’
“We cured the mouse-and this has never been seen with other therapies,” Dr. Osborne noted, adding that other mechanisms of resistance may still develop. Therefore, complete blockade will not be the answer in all cases.
In a collaboration with the HERmark investigators, Dr. Osborne’s group will apply the HERmark assay to their preclinical models and to tumor samples from patients treated with trastuzumab and lapatinib.
“This technology is not ready to be used any time soon, but it is promising, and it will be useful if these results hold up in additional studies,” he said.
How it works
The VeraTag assay is a novel proximity-based technology. To measure total HER2 (H2T), a monoclonal antibody specific for a unique epitope of HER2 (Ab8) is conjugated to a fluorescein VeraTag reporter (Pro11) by means of a cleavable tether.
This antibody is paired with a biotinylated second antibody directed against the C terminus of HER2 (Ab15). A molecular scissors is subsequently added and bound to the biotinylated antibody.
Upon irradiation with red light, the molecular scissors liberates singlet O2. The free radicals cleave all thioether bonds within approximately 200 nM, releasing Pro11. The signal can then be collected and analyzed on a capillary electrophoresis array. Each VeraTag reporter is designed with a unique charge-mass ratio and can thus be identified and quantitated by comparison to assay standards.