New Standards Proposed for Treating Aggressive NHL- Age a Factor
HOMBURG, Germany-Afterconducting trials comparingCHOP (cyclophosphamide [Cytoxan,Neosar], doxorubicin HCl,vincristine [Oncovin], prednisone)with and without etoposide andvarying time intervals, the GermanHigh-Grade Non-Hodgkin’s LymphomaStudy Group concluded thatCHOP plus etoposide is the newstandard regimen for younger patientswith low-risk non-Hodgkin’slymphoma (NHL), and CHOP at2-week intervals is the new standardregimen for aggressive NHL in olderpatients.
HOMBURG, Germany-Afterconducting trials comparingCHOP (cyclophosphamide [Cytoxan,Neosar], doxorubicin HCl,vincristine [Oncovin], prednisone)with and without etoposide andvarying time intervals, the GermanHigh-Grade Non-Hodgkin's LymphomaStudy Group concluded thatCHOP plus etoposide is the newstandard regimen for younger patientswith low-risk non-Hodgkin'slymphoma (NHL), and CHOP at2-week intervals is the new standardregimen for aggressive NHL in olderpatients.The two trials had the same objectives,though with different agegroups: to improve treatment resultsin patients with aggressive NHL byshortening treatment intervals and/or adding CHOP to etoposide.Among the group of 18- to 60-yearolds,CHOEP (CHOP plusetoposide) achieved the objective.Among the group of 61- to 75-yearolds,shortening the time intervalbetween CHOP treatment coursesfrom 3 to 2 weeks achieved the objective.Michael G. Pfreundschuh,MD, of Medizinische Klinik I,Universitt des Saarlands, Hom-burg, Germany, reported the resultsat the 43rd Annual Meeting of theAmerican Society of Hematology(abstracts 3026 and 3027).Same Regimens and
End Points
In both studies, patients wererandomized to receive six cycles ofCHOP(21) (CHOP every 3 weeks),CHOP(14) (CHOP every 2 weeks),CHOEP(21) (3-weekly CHOEP), orCHOEP(14) (2-weekly CHOEP). Inboth CHOEP groups, the etoposidewas administered at 100 mg/m2 ondays 1 to 3. Patients in the 2-weeklyregimens also received granulocytecolony-stimulating factor (G-CSF[Neupogen]) starting on day 4.
The primary end point for bothstudies was time to treatment failure.The interim analyses for bothstudies included patients who hadeither follow-up of more than 3months after therapy, or an event(defined as disease progression, relapse,death, or initiation of alternativetreatment).Younger and Low Risk
The younger age group included18- to 60-year-olds with untreatedlow-risk aggressive NHL. Low riskwas defined as normal pretreatmentlactate dehydrogenase (LDH). Inclusioncriteria also included anEastern Cooperative OncologyGroup (ECOG) performance statusof 0 to 3, normal white blood countand platelets, and no major organdysfunction.Dr. Pfreundschuh presented interimdata from July 2001 on 659patients with a median age of 48.Sixty-six percent had no risk factors,according to the age-adjusted InternationalPrognostic Index, 31% hadone risk factor, and 3% had two riskfactors. Twenty-eight percent of patientshad bulky disease, defined asgreater than 7.5 cm, and received 36Gy of radiotherapy to the site. "Allfour regimens had a high relative doseintensity, clearly above 95%," Dr.Pfreundschuh reported.Better Response in Etoposide-
Containing RegimensAt nearly 84%, "the etoposidecontainingregimens had a completeremission rate of 89.3%, vs 83.9%with CHOP," Dr. Pfreundschuh said."In contrast, in this population,which did not have patients with elevatedLDH indicating relatively fastgrowingtumors, the effect of the timeinterval reduction was not significant.Similarly, there is no differencein time to treatment failure betweenthe 2-weekly and 3-weekly regimen,but there is significant difference inthe time to treatment failure withrespect to the addition of etoposide."At a median time of observation of40 months, time to progression was72.8% for CHOEP vs 67.5% forCHOP. "The difference is not judgedsignificant for overall survival," Dr.Pfreundschuh added.Adding etoposide had the greatesteffect-increasing the remissionrate by 17%-among patients withone risk factor. "We are suggestingthat the addition of etoposide compensatesfor one risk factor," Dr.Pfreundschuh said. After 40 months,there was also a 22% difference intime to treatment failure and a 15%difference in overall survival."CHOEP regimens have somewhatmore hematologic toxicities,"Dr. Pfreundschuh reported, and requiredmore transfusions. Therewere no major differences innonhematologic toxicities."We conclude that CHOP plusetoposide is superior to CHOP inyoung low-risk (low-LDH) patients,"Dr. Pfreundschuh said. "Interval reductiondid not improve outcome inpatients with aggressive lymphomaand normal LDH. "CHOEP is nomore toxic than CHOP, is well toleratedin this population of youngerpatients. We should therefore considerCHOEP(21) as the new standardregimen for low-risk (low-LDH)patients."Older and Higher Risk
Patients in the second trial reportedby Dr. Pfreundschuh wereONInot only older (61 to 75 years old)but more likely to have elevatedLDH and advanced disease (50%)and bulky disease (40%) requiringadditional radiotherapy. Other inclusioncriteria were the same as forthe younger patients."Adherence to the protocol wasquite good," Dr. Pfreundschuh reported,"except for the double-intensiveCHOEP(14), where the relativedose dropped to 87%."Interim Analysis
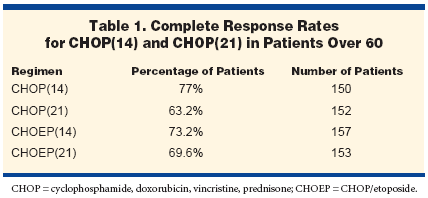
The interim analysis included 612patients, with a median age of 67years. Complete response ratesranged from 63.2% for CHOP(21)to 77% for CHOP(14), with theCHOEP rates in between (Table 1)."It is mostly patients with elevatedLDH, a surrogate model for aggressivegrowth, that profit from thistime interval reduction," Dr.Pfreundschuh said. "Whereas completeresponse rate only increasedby 8% in patients with normal LDH,complete remission rate for patientswith elevated LDH increased from48% to 70%. This translates intosignificant differences in time totreatment failure. With a mediantime of observation of 40 months,difference in time to treatment failureis 14% between the worst arm-CHOP(21)-and the best arm-CHOP(14). This difference is similarfor overall survival."Hematologic toxicities in the 2-weekly regimen were not increasedsignificantly, due to G-CSF. Patientsin the CHOEP regimens had morecycles with platelets below 50,000/μL.New Reference Standard
"In summary, CHOP(14) issuperior to CHOP(21)," Dr.Pfreundschuh said. "It is the best ofthe tested arms in elderly patientswith aggressive NHL. The 2-weeklyregimen is feasible and toxicity isnot increased over CHOP(21) dueto the use of G-CSF. CHOP(14) isour new reference standard for thispopulation."