Tandem Transplants Increase CR, Reduce Treatment Mortality in Multiple Myeloma
SEATTLE-A two-stage procedurethat combines high-dose chemotherapywith autologous stemcell transplantation (SCT) with animmunosuppressive (but notmyeloablative) allogeneic SCT inmultiple myeloma improves completeresponse rate and decreasestreatment-related mortality.
SEATTLE-A two-stage procedurethat combines high-dose chemotherapywith autologous stemcell transplantation (SCT) with animmunosuppressive (but notmyeloablative) allogeneic SCT inmultiple myeloma improves completeresponse rate and decreasestreatment-related mortality.In a plenary presentation at the43rd Annual Meeting of the AmericanSociety of Hematology, DavidG. Maloney, MD, PhD, presenteddata for 41 patients treated with thisapproach (abstract 1822). "The reducedmortality allows treatment ofolder patients. We feel our methodshould now be studied in comparisonto conventional autografting forthe treatment of patients with myeloma,"said Dr. Maloney, who isan associate member of the FredHutchinson Cancer Research Centerin Seattle.Treatment-Related
Mortality an Issue
In introducing this paper,Stephen Mackinnon, MD, pointedto treatment-related mortality as amajor flaw in conventional transplants."We are still losing one outof three of our transplant patientsto transplant-related causes. In addition,conventional transplants aregenerally not applicable to older patients,because age is associated withincreased risk. That is a problem, asmost multiple myeloma patients areelderly." Dr. Mackinnon is a consultantin hematology at UniversityCollege in London.The lower-intensity regimen Dr.Maloney used to prepare patients forthe allogeneic SCT is myelosuppressiverather than myeloablative. It has limitedantitumor efficacy but is immunosuppressiveenough to permit transplantengraftment, according toDr. Mackinnon. The main differencecompared to conventional regimens isin the use of low-dose total-body irradiation.Dr. Maloney said that althoughmyeloablative allogeneic SCT is potentiallycurative for myeloma (due tothe graft-vs-myeloma effect), hisgroup has been concerned about hightransplant-related mortality due toregimen-related toxicities and graftvs-host disease. "The alternative ofhigh-dose therapy with autologousstem cell rescue induces cytoreductionof the disease with a low transplantrelatedmortality, but nearly all patientseventually relapse or developdisease progression," he said.We are losing
one out of three
transplant
patientsTherapy Could Be Curative
The investigators reasoned thatcurative therapy might be possibleif the safety and cytoreduction ofhigh-dose melphalan (Alkeran) andautologous SCT could be combinedwith the graft-vs-myeloma potentialof nonmyeloablative allogeneicSCT from human leukocyte antigen(HLA)-matched sibling donors."By separating the high-dose conditioningregimen from the immunotherapeuticeffect of the allograft,we hoped to decrease the transplantrelatedmortality yet maintain thegraft-vs-myeloma effect," Dr.Maloney said.Median Follow-up
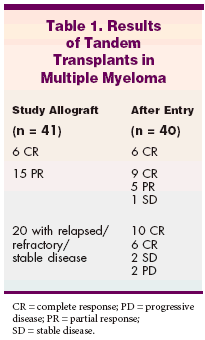
Dr. Maloney reported median 13-month follow-up data on 41 patientswith a median age of 52 years (range:39-71), with previously treated stageII (n = 11) or stage III (n = 30) myelomabut no previous transplant. Forty-nine percent of patients had refractoryor relapsed disease, 36% hadpartial responses to prior treatment,and 15% were in CR at study entry.The patients were given granulocytecolony-stimulating factor (16g/kg/d x 4 days) and stem cellswere collected. Patients then receivedmelphalan at 200 mg/m2 followedby autologous stem cell rescue.At a median of 62 days later (range:40-120 days), patients received 2 Gy oftotal-body irradiation (7 cGy/min), immunosuppressionwith mycophenolatemofetil (CellCept) for 28 days,cyclosporine (Neoral, Sandimmune)(for a minimum of 56 days), and unmodifiedperipheral blood stem cell allograftsfrom HLA-identical siblings.Of the 41 patients, 40 receivednonmyeloablative allogeneic SCT.The majority of patients did not requirehospitalization. Granulocyteand platelet nadirs after allograft were736 cells/μL and 97,000 cells/μL, respectively.Dr. Maloney reported thatall patients had successful engraftment,with medians of 90% of donorT-cell chimerism by day 28 after allograftand 99% by day 84.With median follow-ups of 423 daysafter autologous and 328 days afternonmyeloablative SCT, overall survivalwas 85%, progression-free survivalwas 83%, and 1-year treatment-relatedmortality was 12%. The responserate was 88%, with 61% complete responseand 27% partial response andonly two progressions to date."Patients continue to move frompartial to complete response," Dr.Maloney said (see Table 1).Graft-vs-host disease was the mostserious complication, as 19 of 41 patientsdeveloped acute cases. This included16 patients with grade II, 1 withgrade III, and 2 who developed fatalgrade IV graft-vs-host disease. Fortyfivepercent of patients developedchronic disease requiring therapy.Potent Antitumor Activity
"Despite being used in an older groupof patients with multiple myeloma, thisnovel two-step allografting approach hasdramatically reduced the acute toxicitiesof allogeneic SCT, while maintainingpotent antitumor activity. This studyprovides the rationale for a comparativestudy between this two-step allogeneicapproach and standard autologous SCTfor the therapy of myeloma," Dr.Maloney concluded.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.