Non-Hodgkin's Lymphoma in the Elderly (Part 2: Treatment of Diffuse Aggressive Lymphomas)
As noted in part 1 of this two-part article, non-Hodgkin's lymphoma is one of a few malignancies that have been increasing in incidence over the past several decades. Likewise, these disorders are more common in elderly patients, with a median age of occurrence of 65 years. Therapy in elderly patients may be affected by multiple factors, especially attendent comorbidities. The approaches to management of these patients, with either indolent or aggressive disease processes, have been based on prospective clinical trial results, many of which have included a younger patient population. Fortunately over the past decade, results of treatment trials that have targeted an older patient population have emerged. The disease incidence and treatment approaches for both follicular (part 1) and diffuse aggressive (part 2) histologies in elderly patients are reviewed, as well as the impact of aging on the care of these patients.
As noted in part 1 of this two-part article, non-Hodgkin's lymphoma is one of a few malignancies that have been increasing in incidence over the past several decades. Likewise, these disorders are more common in elderly patients, with a median age of occurrence of 65 years. Therapy in elderly patients may be affected by multiple factors, especially attendent comorbidities. The approaches to management of these patients, with either indolent or aggressive disease processes, have been based on prospective clinical trial results, many of which have included a younger patient population. Fortunately over the past decade, results of treatment trials that have targeted an older patient population have emerged. The disease incidence and treatment approaches for both follicular (part 1) and diffuse aggressive (part 2) histologies in elderly patients are reviewed, as well as the impact of aging on the care of these patients.
As noted in part 1 of this article, which appeared in the August issue of ONCOLOGY (21:1104-1110, 2007), non-Hodgkin's lymphoma (NHL) continues to increase in incidence and is more common in elderly patients. After examining the impact of aging on the disease and exploring prognostic factors in this setting, part 1 reviewed the treatment of patients with follicular lymphoma. Here, part 2 will address the treatment of diffuse aggressive lymphomas in older patients.
Therapy of Diffuse Aggressive Lymphomas
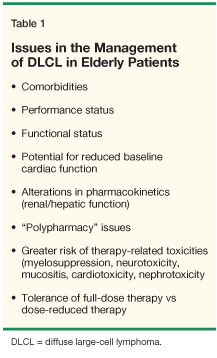
The diffuse aggressive lymphomas include a variety of histologic subtypes, among which the diffuse large-cell histology (DLCL) is the most common. In elderly patients, B-cell DLCL (DLBCL) is the most common subtype, accounting for 50% of all cases of NHL in these patients.[1,2] Among those aged 65 to 75 years, 50% will achieve a complete response (CR) with conventional therapies, with a 5-year disease-free survival (DFS) of about 33%. However, the CR rate drops to 40% for patients older than 75 years, with a 16-month median response duration (Table 1).[1]
Approach to Limited-Stage Disease
Approximately 30% of patients with diffuse aggressive NHL will have limited-stage disease. In an initial report of an Eastern Cooperative Oncology Group study of patients with limited-stage disease with 5-year follow-up, therapy with three cycles of CHOP (cyclophosphamide, doxorubicin HCl, vincristine [Oncovin], prednisone) followed by involved-field radiotherapy resulted in an improved progression-free (PFS) and overall survival (OS) and less cardiotoxicity, compared to treatment with eight cycles of CHOP.[3] However, in a later report of this study with a median follow-up of 10 years, the progression-free and overall survival curves for the two treatment arms appeared to come together between 7 and 9 years.[4] Using a similar approach, the Vancouver group found that the 10-year DFS was similar to that of younger patients.[2]
In another recent report, a series of 576 patients over 60 years old with localized stage I/II diffuse aggressive NHL were randomized to therapy with either CHOP alone or CHOP plus involved-field radiation therapy. With a median follow-up period of 7 years among the two groups, no difference was found in either event-free survival (EFS) or OS.[5]
Management of Advanced-Stage Disease
The majority of patients with diffuse aggressive NHL have advanced-stage disease, regardless of age. Therapy with CHOP for many years was the standard regimen for these patients, with cure rates of 25% to 30%, compared to 50% to 60% of younger patients, and a toxic death rate of 1%.[2,6,7] This was based on the results of an intergroup trial in which CHOP was compared to other combination regimens (m-BACOD [methotrexate, bleomycin, doxorubicin (Adriamycin), cyclophophamide, vincristine, dexamethasone], ProMACE-CytaBOM [prednisone, methotrexate, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, methotrexate], MACOP-B [methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin]), showing no significant difference in efficacy (CR, OS, or PFS) but fewer adverse events with CHOP.[8]
• Impact of Age-The impact of age on outcome has been examined in multiple series, albeit generally in a retrospective manner. In a series of patients aged ≥ 70 years treated with CHOP with no initial dose adjustment, the CR rate was comparable to that of younger patients, but more treatment-related complications were seen, including 30% treatment-related deaths (primarily sepsis).[9]
Vose et al reported the results of CAP/BOP therapy (cyclophophamide, doxorubicin, procarbazine, bleomycin, vincristine, prednisone) in 157 patients (112 > 60 years old), in which patients ≥ 70 years old had a 33% dose reduction in myelosuppressive drugs.[10] The response rate was similar in patients under or over age 60 (76% vs 61%, P = .18), as was the CR rate (76% vs 60%, P = .12), DFS, remission duration, and treatment toxicities. However, 5-year survival was shorter in patients over 60 compared to younger patients (34% vs 62%, P = .01), primarily related to intercurrent causes of death, especially late cardiovascular deaths.
In a series of 177 patients with DLCL treated with CHOP-based therapy, Gottlieb et al found a lower CR rate in patients over 70 than in younger patients (27% vs 53%, P = .01).[11] However, these older patients were less likely to receive full-dose therapy. Grogan found no correlation of age with outcome in a study in which 67 patients under age 65 and 60 patients at least 65 years of age received standard-dose CHOP or m-BACOD.[12] Response rates were comparable for older (95%/65% CR) and younger (92%/76% CR) patients, as were 3-year OS (59% vs 62%), DFS (74% vs 82%), and toxic death rate.
In a multivariate analysis of elderly patients treated with CHOP, Gomez et al found that poor performance status (PS) was the only risk factor for treatment-related death.[13] Age ≥ 60 years was prognostic for outcome in Solal-Celigny et al's series of 73 patients receiving anthracycline-based therapy, with a lower CR rate (24% vs 72%), shorter median survival (18 vs 48 months), and lower 5-year survival (18% vs 47%) in these older patients, compared to those < 60 years of age.[14] Lastly, Tirelli et al found that severe and lethal toxicities were more common in patients aged at least 70 years who were treated with more aggressive regimens.[15]
• Impact of Delivered Dose-The impact of delivered dose on outcome in the elderly has also been evaluated in various studies. Dixon et al found that in DLCL patients over 65 who received an initial 50% dose reduction in CHOP-like regimens, the CR rate was lower (37% vs 65%) and median survival was shorter (16 vs > 101 months) than in patients < 40 years of age.[16] However, the outcome of older patients who received full-dose therapy in this trial was comparable to that of the younger patients.
O'Connell et al also recommended full-dose therapy for older patients, based on a series of 141 patients ≥ 60 years of age treated with COPA (cyclophophamide, vincristine, prednisone, doxorubicin), in which full-dose therapy resulted in a higher CR rate (49% vs 33%, P = .07), as well as prolonged survival (P = .02), compared to patients who had a 50% dose reduction.[17] Neutopenia was more common with full-dose than dose-reduced therapy (16% vs 10%), as were severe infections (5% and 2%).
The feasibility of delivering full-dose CHOP therapy to elderly patients has been advocated.[18-20] Campbell et al found that the subset of patients ≥ 65 years old with favorable PS (< 2) and few comorbidities can tolerate full-dose CHOP without growth factor support.[18] Likewise, Jacobson et al reported that full-dose CHOP with "preemptive" growth factor support could be administered to patients > 60 years old, with a high delivered dose intensity, a low rate of neutropenia and neutropenic fevers, and no significant cardiac toxicity or treatment-related mortality.[20]
• Strategies to Reduce Toxicity-A variety of approaches have been taken to maximize outcome with reduced toxicity in the elderly population. The administration of dose-intensive therapy over a short period of time has been examined in a variety of single-arm phase II trials in elderly patients.[6,21-30] In general, inferior results are seen if the regimen is too toxic.
Zinzani et al reported on the results of therapy with VNCOP-B (etoposide [VePesid], mitoxantrone [Novantrone], cyclophosphamide, vincristine, prednisone, bleomycin) in an elderly patient population.[25-27] In 158 patients ≥ 60 years of age treated with VNCOP-B alone or with granulocyte colony-stimulating factor (G-CSF, Neupogen), the frequency of neutropenia was reduced with G-CSF support compared to controls (23% vs 56%, P = .00005), as was the incidence of clinically relevant infections (5% vs 21%, P = .004).[26] The delivered dose intensities were comparable.
This Italian multicenter group subsequently treated 350 patients over 60 with VNCOP-B, with 71% of patients receiving G-CSF.[27] The response rate was 83% (58% CR), with CR rates similar in patients aged 60 to 69, 70 to 79, and over 80. At 5 years, the relapse-free survival (RFS) was 65%, and OS was 49%. In multivariate analysis, factors prognostic for longer OS or RFS were localized disease and a good PS. Martelli et al reported the use of P-VABEC (prednisone, vincristine, doxorubicin, bleomycin, etoposide, cyclophosphamide) in 60 patients > 60 years of age, with a CR rate of 75%, projected 2-year OS of 64%, and DFS of 57%, and minimal hematologic toxicity.[28] Use of this regimen in other series has led to similar results.[29,30]
Administration of the non-anthracycline-containing regimen COPP (cyclophosphamide, vincristine, procarbazine, prednisone) was found to result in a lower CR rate and shorter survival compared with anthracycline-containing regimens.[31] Therapy with oral etoposide, alone or in combination, results in moderate myelosuppression that may be prolonged.[32-34] In an effort to reduce cardiotoxicity, mitoxantrone has been utilized in a variety of regimens.[35-40] In general, this has resulted in potentially fewer cardiac complications, but significant myelotoxicity. Other anthracyclines such as idarubicin and epirubicin have been utilized in CHOP or P-VABEC-like regimens with acceptable toxicities.[41-43]
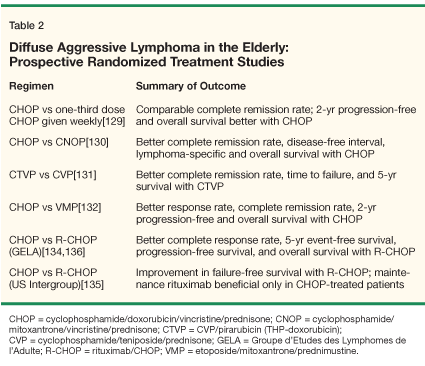
• Major Trials With CHOP-Like Regimens-Over the past decade, several large prospective randomized treatment trials have been conducted in the elderly population (Table 1).[44-51] The results of these randomized studies have had a significant impact on the management of lymphomas in this population. While the first five trials utilized CHOP-like regimens,[44-48] the following two trials established the role of R-CHOP (rituximab [Rituxan] plus CHOP) as the standard of care for patients with DLCL.[49-51]
In the randomized phase II reported by Meyer et al, full-dose CHOP was compared with one-third-dose "chop" given weekly in 38 patients ≥ 65 years of age.[44] The CR rate was comparable with CHOP and chop (68% vs 74%, P = NS), as was the 2-year PFS (57% vs 46%, P = NS). However, the 2-year OS was superior with CHOP (74% vs 51%, P = .05). Although more myelosuppression occurred with CHOP, it was not associated with significant dose reductions or delays.
The use of mitoxantrone in place of doxorubicin was examined by the Dutch Cooperative Hematology Group in a phase III trial for which patients over 60 were randomized to therapy with CHOP vs CNOP (cyclophosphamide, mitoxantrone, vincristine, prednisone).[45] In the 145 patients assessable for response, CHOP resulted in a superior outcome compared to CNOP, in terms of CR rate (49% vs 31%, P = .03), median disease-free interval (27 vs 15 months), lymphoma-specific survival (P = .034), and OS (P = .029). Likewise, more partial responders (PR) after three cycles of therapy subsequently achieved a CR with CHOP than with CNOP. Dose intensity was ≥ 90% for both regimens, and toxicities were similar, although less nausea and alopecia were seen with CNOP.
The results of the Groupe d'Etudes des Lymphomes de l'Adulte (GELA) trial in which 453 previously untreated patients at least 70 years old were randomized to CTVP (cyclophosphamide, pirarubicin [THP-Adriamycin], teniposide [Vumon], prednisone) or CVP (cyclophosphamide, teniposide, prednisone) were reported by Bastion et al.[46] This was a high-risk population, with advanced-stage disease in 67%, more than two extranodal disease sites in 68%, bulky disease in 32%, marrow involvement in 25%, and an elevated lactate dehydrogenase (LDH) in 59%. Outcome was superior with CTVP as compared to CVP, with regard to CR rate (47% vs 32%, P = .0001), median time to treatment failure (TTF, 7 vs 5 months, P < .05), and 5-year OS (26% vs 19%, P < .05). Although the investigators saw more leukopenia, thrombocytopenia, infections, alopecia, and mucositis in patients receiving CTVP, no differences in cardiac complications or death during treatment were present among the two regimens. In multivariate analysis, factors prognostic for longer survival or TTF were localized stage, good PS, normal serum LDH, and serum albumin ≥ 3.5 mg/dL.
Tirelli et al reported the results of a European Organisation for Research and Treatment of Cancer (EORTC) Lymphoma Cooperative Study Group trial in which DLCL patients over age 70 with stage II-IV disease were randomized to six cycles of CHOP or VMP (etoposide, mitoxantrone, prednimustine [Sterecyt]).[47] Patients with a good PS received full-dose therapy, whereas a reduced dose was used for poor-PS patients. In 120 patients assessable for response, results seen with CHOP were superior to those with VMP, in terms of overall response rate (77% vs 50%, P = .01), CR rate (45% and 27%, P = .06), 2-year PFS (55% vs 25%, P = .002), and 2-year OS (65% vs 30%, P = .004). Although hematologic toxicities were comparable, the researchers found significantly more alopecia, neurotoxicity, and gastrointestinal toxicity with CHOP. Cardiotoxicities were also more common with CHOP (14% vs 8%, P = NS). The toxic death rate with CHOP was only 1.6%, which may have been related to the PS-based dose reductions.
The impact of the addition of etoposide to CHOP therapy (CHOEP) in elderly patients was examined by the German High-Grade Non-Hodgkin's Lymphoma Study Group.[48] Approximately 700 patients over 60 were randomized to receive therapy with biweekly CHOP (CHOP-14), CHOEP-14, traditional CHOP (CHOP-21), or CHOEP-21. Myeloid growth factor support was included in the biweekly regimens. Both EFS and OS were superior with the CHOP-14 regimen, compared to therapy with CHOP-21. Toxicity was significantly greater with either of the etoposide-containing regimens. As such, the authors recommended that CHOP-14 should be considered as the new standard therapy for these elderly patients.
• Major Trials With Rituximab-In the subsequent generation of phase III trials, the impact of the addition of rituximab to CHOP therapy was examined in patients with previously untreated diffuse aggressive B-cell NHL, with two studies being done in elderly populations.[49-51] The results of these studies established the role of R-CHOP as initial therapy for previously untreated patients with DLBCL, both in older patients and in younger patients with favorable prognostic characteristics.
In the GELA study reported by Coiffier et al, 399 patients aged 60 to 80 years with previously untreated DLCL were randomized to therapy with eight cycles of either CHOP or R-CHOP.[49] Rituximab, 375 mg/m2, was administered on day 1 of each cycle of R-CHOP. Results of this trial with 5 years of follow-up were recently reported.[51] The outcome of R-CHOP therapy remains superior to CHOP, with regard to CR rate (76% vs 63%, P = .005), as well as 5-year EFS, PFS, and DFS. However, there was a statistically significant improvement in 5-year OS only among low-risk, but not high-risk, International Prognostic Index (IPI) patients. In patients with a high-risk IPI score, a higher mortality rate was seen due to diseases unrelated to NHL. A lower relapse rate was found in the patients treated with R-CHOP than in those given CHOP. The majority of CHOP-treated patients who relapsed died of disease progression. It was also found that patients with concomitant diseases or a poor PS at diagnosis were more likely to die in CR.
In the US Intergroup trial (Eastern Cooperative Oncology Group/Southwestern Oncology Group 4494, Cancer and Leukemia Group B 9793), 632 previously untreated patients with diffuse aggressive B-cell NHL were randomized to therapy with R-CHOP or CHOP, for a maximum of eight cycles.[50] Patients also received two doses of rituximab, 375 mg/m2, prior to cycle 1, then a single dose given prior to cycles 3, 5, and 7. Thus, patients received a maximum of five doses of rituximab in this study, in contrast to eight doses in the GELA study. Another important difference in the US Intergroup trial was the 22 trial design, such that patients who achieved a response to initial therapy with either R-CHOP or CHOP went onto a second randomization of maintenance therapy with rituximab (375 mg/m2) given weekly for 4 weeks, repeated every 6 months for 2 years, or observation.
The initial objectives of this study were to compare the results of induction therapy with either R-CHOP or CHOP, and to examine the impact of maintenance therapy with rituximab vs observation. However, the use of maintenance rituximab confounded these proposed analyses, and therefore a weighted analysis of the study was performed. The overall response rate to induction therapy was comparable among the two arms (77% with R-CHOP, 76% with CHOP). Although there was a trend toward an improved failure-free survival (FFS) with R-CHOP, no difference in OS has yet been seen, with a median follow-up of 3.5 years.
Several important findings were found with the weighted analyses. Among the responding patients who were randomized to maintenance-phase observation, an improved FFS and OS was found in patients who received R-CHOP as compared to CHOP. The impact of maintenance rituximab therapy varied by the induction treatment given. For patients who received CHOP, the 2-year FFS was prolonged by the administration of maintenance rituximab compared to observation (74% vs 45%). However in patients who received R-CHOP, the use of maintenance rituximab resulted in no improvement in the 2-year OS compared to observation (87% vs 85%, respectively). Thus, maintenance rituximab therapy in responding patients benefited only those patients who had initially received CHOP, with no incremental benefit seen in R-CHOP-treated patients.
The use of R-CHOP therapy in diffuse aggressive B-cell NHL patients, with no maintenance therapy, has also been examined in other series.[52,53] In a phase II study by Vose et al, 33 previously untreated patients received six cycles of R-CHOP, with rituximab, 375 mg/m2, administered 2 days before each cycle of CHOP.[52] Of the 33 patients, 10 were older than age 60. The overall response rate was 94% (61% CR, 33% PR), and with a median follow-up of 26 months, the median duration of response and time to progression had not been reached.
The British Columbia experience with R-CHOP was reported by Sehn et al.[53] Patients with previously untreated DLBCL received therapy with CHOP prior to a mandate in 2001 for the addition of rituximab to CHOP therapy for patients diagnosed thereafter. In these 292 patients, R-CHOP therapy resulted in an improved 2-year PFS (69% vs 51%, P = .002) and 2-year OS (78% vs 52%, P < .0001), compared to treatment with CHOP. Age ≥ 60 years was predictive for a shorter PFS and OS in this series.
Use of Myeloid Growth Factors
Approaches to improve the outcome of elderly patients with diffuse aggressive NHL have focused on ameliorating myelotoxicity with supplemental therapies such as myeloid growth factors, as neutropenia and subsequent infectious complications are more common in this population. Multiple studies have found that the addition of myeloid growth factors results in a decreased frequency of infections but, in general, no significant improvement in outcome parameters.[54-62]
In the phase II trial conducted by Gomez et al, patients received therapy with CHOP plus granulocyte-macrophage colony-stimulating factor (GM-CSF, Leukine). These investigators found that a nadir absolute neutrophil count < 500/mm3 occurred more often in patients ≥ 70 years of age, compared to patients aged 61 to 69 years (73% vs 24%, P = .00001), as did febrile neutropenia (42% vs 8%, P < .0001).[60]
In a series reported by Zinzani et al, previously untreated patients aged 60 or older with high-grade NHL received combination anthracycline-based chemotherapy therapy alone or with G-CSF support. The incidence of grade 3/4 myelosuppression was more common with no growth factor support (56% vs 5%, P < .001), as was infection (21% vs 5%, P = .004).[61] Likewise, Bjorkholm et al found that grade 3/4 neutropenia was less common with G-CSF support (62% vs 91%, P < .001), as was the incidence of infection (33% vs 50%, P = .001), in a large series of older patients treated with either CHOP or CNOP, with or without G-CSF.[62]
• Large Randomized Trials-Three large randomized studies have been reported in which the utility of adding myeloid growth factors has been examined in older patients with DLCL receiving anthracycline-based regimens.[63-65] In the trial by Doorduijn et al, 389 previously untreated patients aged 65 to 90 (median = 72) with aggressive NHL were randomized to therapy with CHOP or CHOP plus G-CSF.[64] Although the relative dose intensity of cyclophosphamide was higher in patients receiving CHOP plus G-CSF than CHOP alone (median = 96.3% vs 93.9%, P = .01), as was that of doxorubicin (median = 95.4% vs 93.3%, P = .04), there was no difference in the CR rate, 5-year OS rate, and incidence of grade 3/4 infectious complications between the two arms.
Osby et al reported the results of the Nordic Lymphoma Group trial involving 455 patients aged 60 to 86 (median = 71) with aggressive NHL.[65] In this trial, patients were randomized to initial therapy with either CHOP or CNOP, given either alone or with G-CSF. Secondary prophylaxis was not allowed in patients randomized to either treatment arm with no growth factor support. Therapy with CHOP provided a superior outcome to that seen with CNOP, regardless of G-CSF usage. Although a relative dose intensity ≥ 90% was achieved more often in the treatment arms with G-CSF support, growth factor use had no impact on the CR rate, TTF, or OS. In contrast to the Doorduijn trial, however, Osby and colleagues found a reduction in the occurrence of grade 4 neutropenia and neutropenic infections with growth factor support.
Lastly, in the phase II trial reported by Gomez et al, previously untreated patients received CHOP with GM-CSF support. Treatment-related mortality was 18% in patients over age 70, compared to 0% in the younger cohort.[63]
Predictors of Treatment-Related Complications
The identification of disease- and patient-related characteristics that may be predictive for the occurrence of therapy-related neutropenia and febrile neutropenia is of importance, as the prospective identification of patient subgroups at high risk for these complications would enable physicians to administer myeloid growth factors in the most useful and cost-effective manner. Pretreatment variables identified in one study as predictive for first-cycle CHOP febrile neutropenia included serum albumin ≤ 3.5 mg/dL, an elevated LDH, and marrow involvement at presentation, but not age or PS.[66] Patients with either a low albumin level, or a normal albumin but an elevated LDH and marrow involvement, had a 72% incidence of febrile neutropenia, compared to 17% in patients with either marrow involvement with a normal albumin and LDH, or an elevated LDH with a normal albumin and no marrow involvement.
Likewise, in the US Intergroup study, there was no impact of gender, PS, anemia (hemoglobin < 12 g/dL), elevated LDH, marrow involvement, or IPI risk score on subsequent growth factor utilization. However, the "older" patients in this study (age > 65 years) were more likely to have received growth factor support than the "younger" patients (age 60-64 years).[152] Factors predictive for the development of first-cycle febrile neutropenia with CHOP or R-CHOP therapy in this trial included advancing age, poor PS (PS 2 or 3), anemia (hemoglobin < 12 g/dL), elevated LDH, and a high-intermediate or high-risk IPI score.
In another series of intermediate-grade NHL patients receiving initial CHOP therapy, risk factors predictive for hospitalization for febrile neutropenia included age ≥ 65 years (P < .0001), the presence of renal disease (P = .0004), a planned average relative dose intensity > 80% (P = .0083), and no myeloid growth factor administration during days 1 through 5 of the treatment cycle (P = .0237).[68,69] In a recent meta-analysis, myeloid growth factor use in patients with therapy-related febrile neutropenia was found to reduce both the length of hospitalization and neutrophil recovery time.[70] The cost-effectiveness of growth factor usage in older patients receiving CHOP-like therapy has also been examined.[71-77]
Conclusions
The approach to management of elderly patients with NHL will continue to be a significant issue, given that the median age of patients with this disorder is over 60, and that the proportion of elderly patients in the population is increasing. The presence of comorbidities in these patients has an impact on therapies that can be offered to these patients. Over the past decade, prospective clinical trials have been designed to address the treatment not only of younger, healthier patients, but also of these older patients. Both the efficacy of therapy and the toxicity profile of such treatment need to be evaluated in these older patients. The results of these studies have been crucial in defining optimal treatment approaches in this population.
Disclosures:
The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Ballester OF, Moscinski L, Spiers A, et al: Non-Hodgkin's lymphoma in the older person: a review. J Am Geriatr Soc 41:1245-1254, 1993.
2. Connors JM, O'Reilly SE: Treatment considerations in the elderly patient with lymphoma. Hematol Oncol Clin North Am11:949-960, 1997.
3. Miller TP, Dahlberg S, Cassaday JR, et al: Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med 339:21-26, 1998.
4. Miller TP, LeBlanc M, Spier CM, et al: CHOP alone compared to CHOP plus radiotherapy for early stage aggressive non-Hodgkin's lymphoma: Update of the Southwest Oncology Group (SWOG) randomized trial (abstract 3024). Blood 98:724a, 2001.
5. Bonnet C, Fillet G, Mournier N, et al: CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: A study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 25:787-792, 2007.
6. O'Reilly S, Connors JM, Macpherson N, et al: Malignant lymphomas in the elderly. Clin Geriatr Med 13:251-263, 1997.
7. McKelvey EM, Gottlieb JA, Wilson HE, et al: Hydroxy-daunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer 38:1484-1493, 1976.
8. Fisher RI, Gaynor ER, Dahlberg S, et al: Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med 328:1002-1006, 1993.
9. Armitage JO, Potter JF: Aggressive chemotherapy for diffuse histiocytic lymphoma in the elderly: increased complications with advancing age. J Am Geriatr Soc 32:269-273, 1984.
10. Vose JM, Armitage JO, Weisenburger DD, et al: The importance of age in survival of patients treated with chemotherapy for aggressive non-Hodgkin's lymphoma. J Clin Oncol 6:1838-1844, 1988.
11. Gottlieb AJ, Anderson JR, Ginsberg SJ, et al: A randomized comparison of methotrexate dose and the addition of bleomycin to CHOP therapy for diffuse large cell lymphoma and other non-Hodgkin's lymphomas. Cancer 66:1888-1896, 1990.
12. Grogan L, Corbally N, Dervan PA, et al: Comparable prognostic factors and survival in elderly patients with aggressive non-Hodgkin's lymphoma treated with standard-dose adriamycin-based regimens. Ann Oncol 5(suppl 2):S47-S51, 1994.
13. Gomez H, Hidalgo M, Casanova L, et al: Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin's lymphoma: Results of a multivariate analysis. J Clin Oncol 16:2065-2069, 1998.
14. Solal-Celigny P, Chastang C, Herrera A, et al: Age as the main prognostic factor in adult aggressive non-Hodgkin's lymphoma. Am J Med 83:1075-1079, 1987.
15. Tirelli U, Zagonel V, Serraino D, et al: Non-Hodgkin's lymphomas in 137 patients aged 70 years or older: a retrospective European Organization for Research and Treatment of Cancer Lymphoma Group study. J Clin Oncol 6:1708-1713, 1988.
16. Dixon DO, Neilan B, Jones SE, et al: Effect of age on therapeutic outcome in advanced diffuse histiocytic lymphoma: The Southwest Oncology Group experience. J Clin Oncol 4:295-305, 1986.
17. O'Connell JD, Harrington DP, Johnson GJ, et al: Initial chemotherapy doses for elderly patients with malignant lymphoma. J Clin Oncol 4:1418, 1986.
18. Campbell C, Sawka C, Franssen E, et al: Delivery of full dose CHOP chemotherapy to elderly patients with aggressive non-Hodgkin's lymphoma without G-CSF support. Leuk Lymphoma 35:119-127, 1999.
19. Sonneveld P, Huijgens SP, Hagenbeek A: Dose reduction is not recommended for elderly patients undergoing chemotherapy for non-Hodgkin lymphoma. Ned Tijdschr Geneeskd 143:418-419, 1999.
20. Jacobson JO, Grossbard M, Schulman N, et al: CHOP chemotherapy with preemptive granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin's lymphoma: A dose-intensity analysis. Clin Lymph 1:211-217, 2000.
21. Klimo P, Connors JM: MACOP-B chemotherapy for the treatment of diffuse large cell lymphoma. Ann Intern Med 102:596-602, 1985.
22. O'Reilly SE, Klimo P, Connors JM: Low-dose ACOP-B and VABE: Weekly chemotherapy for elderly patients with advanced-stage diffuse large-cell lymphoma. J Clin Oncol 9:741-747, 1991.
23. O'Reilly SE, Connors JM, Howdle S, et al: In search of an optimal regimen for elderly patients with advanced-stage diffuse large-cell lymphoma: results of a phase II study of P/DOCE chemotherapy. J Clin Oncol 11:2250-2257, 1993.
24. McMaster ML, Johnson DH, Greer JP, et al: A brief-duration combination chemotherapy for elderly patients with poor-prognosis non-Hodgkin's lymphoma. Cancer 67:1487-1492, 1991.
25. Zinzani PL, Bendani M, Gherlinzoni F: VNCOP-B regimen in the treatment of high-grade non-Hodgkin's lymphoma in the elderly. Haematologica 78:378-382, 1993.
26. Zinzani PL, Pavone E, Storti S, et al: Randomized trial with or without granulocyte colony-stimulating factor as adjunct to induction VNCOP-B treatment of elderly high-grade non-Hodgkin's lymphoma. Blood 11:3974-3979, 1997.
27. Zinzani PL, Storti S, Zaccaria A, et al: Elderly aggressive-histology non-Hodgkin's lymphoma: First-line VNCOP-B regimen experience on 350 patients. Blood 94:33-38, 1999.
28. Martelli M, Guglielmi C, Coluzzi S, et al: P-VABEC: A prospective study of a new weekly chemotherapy regimen for elderly aggressive non-Hodgkin's lymphoma. J Clin Oncol 11:2362-2369, 1993.
29. Caracciolo F, Petrini M, Capochiani E, et al: Third generation chemotherapy with P-VABEC for aggressive non-Hodgkin's lymphomas of the elderly. Leuk Lymphoma 11:115-118, 1993.
30. Caracciolo F, Petrini M, Capochiani E, et al: Alternating chemotherapy regimen (P-VABEC) for intermediate and high-grade non-Hodgkin's lymphoma of the middle aged and elderly. Hematol Oncol 12:185-192, 1994.
31. Liang R, Todd D, Chan TK, et al: COPP chemotherapy for elderly patients with intermediate and high grade non-Hodgkin's lymphoma. Hematol Oncol 11:43-50, 1993.
32. Hainsworth JD: Chronic administration of etoposide in the treatment of non-Hodgkin's lymphoma. Leuk Lymph 10:65-72, 1993.
33. Young WA, Greco FA, Greer JP, et al: Aggressive non-Hodgkin's lymphoma in the elderly: An effective, well-tolerated treatment regimen containing extended-schedule etoposide. J Natl Cancer Inst 86:1346-1347, 1994.
34. Novitzky N, King HS, Johnson C, et al: Treatment of aggressive non-Hodgkin's lymphoma in the elderly. Am J Hematol 49:103-108, 1995.
35. Sonneveld P, Michiels JJ: Full dose chemotherapy in elderly patients with non-Hodgkin's lymphoma: A feasibility study using a mitoxantrone containing regimen. Br J Cancer 62:105-108, 1990.
36. Bessell EM, Coutts A, Fletcher J, et al: Non-Hodgkin's lymphoma in elderly patients: A phase II study of MCOP chemotherapy in patients aged 70 years or over with intermediate or high-grade histology. Eur J Cancer 30A:1337-1341, 1994.
37. Salvagno L, Contu A, Bianco A, et al: A combination of mitoxantrone, etoposide and prednisone in elderly patients with non-Hodgkin's lymphoma. Ann Oncol 3:833-837, 1992.
38. Goss P, Burkes R, Rudinskas L, et al: A phase II trial of prednisone, oral etoposide, and novantrone (PEN) as initial treatment of non-Hodgkin's lymphoma in elderly patients. Leuk Lymphoma 18:145-152, 1995.
39. Ansell SM, Falkson G: A phase II trial of a chemotherapy combination in elderly patients with aggressive lymphoma. Ann Oncol 4:172-175, 1993.
40. Tirelli U, Zagonel V, Errante D, et al: A prospective study of a nnew combination chemotherapy regimen in patients older than 70 years with unfavorable non-Hodgkin's lymphoma. J Clin Oncol 10:228-236, 1992.
41. Zagonel V, Tirelli U, Carbone A, et al: Combination chemotherapy specifically devised for elderly patients with unfavourable non-Hodgkin's lymphoma. Cancer Invest 8:575-580, 1990.
42. Bertini M: Therapeutic strategies in intermediate grade lymphomas in elderly patients. The Italian Multiregional non-Hodgkin's Lymphoma Study Group (IMRNHLSG). Hematol Oncol 11(suppl 1):52-58, 1993.
43. Morra E, Gargantini L, Nosari A, et al: Treatment of patients with high-grade non-Hodgkin's lymphoma aged over 70 years with an all-oral regimen combining idarubicin, etoposide and alkylators. Crit Rev Oncol Hematol 35:95-100, 2000.
44. Meyer RM, Browman GP, Samosh ML, et al: Randomized phase II comparison of standard CHOP with weekly CHOP in elderly patients with non-Hodgkin's lymphoma. J Clin Oncol 13:2386-2393, 1995.
45. Sonneveld P, de Ridder M, van der Lelie H, et al: Comparison of doxorubicin and mitoxantrone in the treatment of elderly patients with advanced diffuse non-Hodgkin's lymphoma using CHOP versus CNOP chemotherapy. J Clin Oncol 13:2530-2539, 1995.
46. Bastion Y, Blay JY, Divine M, et al: Elderly patients with aggressive non-Hodgkin's lymphoma: Disease presentation, response to treatment, and survival-a Groupe d'Etude des Lymphomes de l'Adulte study on 453 patients older than 69 years. J Clin Oncol 15:2945-2953, 1997.
47. Tirelli U, Errante D, Van Glabbeke M, et al: CHOP is the standard regimen in patients ≥ 70 years of age with intermediate-grade and high-grade non-Hodgkin's lymphoma: Results of a randomized study of the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Study Group. J Clin Oncol 16:27-34, 1998.
48. Pfreundschuh M, Trumper L, Kloess M, et al: Two-weekly of 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: Results of the NHL-B2 trial of the DSHNHL. J Clin Oncol 104:634-641, 2004.
49. Coiffier B, Lepage E, Briere J, et al: CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235-242, 2002.
50. Habermann TM, Weller EA, Morrison VA, et al: Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 24:3121-3127, 2006.
51. Feugier P, Van Hoof A, Sebban C, et al: Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A Study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 23:4117-4126, 2005.
52. Vose JM, Link BK, Grossbard ML, et al: Phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive non-Hodgkin's lymphoma. J Clin Oncol 19:389-397, 2001.
53. Sehn LH, Donaldson J, Chhanabhai M, et al: Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 23:5027-5033, 2005.
54. Monfardini S, Sacco C, Serraino D, et al: Effects of G-CSF in patients with non-Hodgkin's lymphomas (NHL) older than 70 years (abstract 611). Ann Oncol 7(suppl 3):164, 1996.
55. Bertini M, Freilone R, Vitolo U, et al: P-VEBEC: a new 8-weekly schedule with or without rG-CSF for elderly patients with aggressive non-Hodgkin's lymphoma (NHL). Ann Oncol 5:895-900, 1994.
56. Niitsu N, Umeda M: Usefulness of COP-BLAM therapy with concomitant G-CSF in elderly patients with non-Hodgkin's lymphoma in comparison with patients not given G-CSF. Nippon Ronan Igakkai Zasshi 32:410-415, 1995.
57. Bertini M, Freilone R, Vitolo U, et al: The treatment of elderly patients with aggressive non-Hodgkin's lymphoma: feasibility and efficacy of an intensive multidrug regimen. Leuk Lymphoma 22:483-493, 1996.
58. Guerci A, Lederlin P, Reyes F, et al: Effect of granulocyte colony-stimulating factor administration in elderly patients with aggressive non-Hodgkin's lymphoma treated with a pirarubicin-combination chemotherapy regimen. Ann Oncol 7:966-969, 1996.
59. Niitsu N, Umeda M: THP-COPBLM (pirarubicin, cyclophosphamide, vincristine, prednisone, bleomycin and procarbazine) regimen combined with granulocyte colony-stimulating factor (G-CSF) for non-Hodgkin's lymphoma in elderly patients: A prospective study. Leukemia 11:1817-1820, 1997.
60. Gómez H, Mas L, Casanova L, et al: Elderly patients with aggressive non-Hodgkin's lymphoma treated with CHOP chemotherapy plus granulocyte-macrophage colony-stimulating factor: Identification of two age subgroups with differing hematologic toxicity. J Clin Oncol 16:2352-2358, 1998.
61. Zinzani PL, Pavone E, Storti S, et al: Randomized trial with or without granulocyte colony-stimulating factor as adjunct to induction VNCOP-B treatment of elderly high-grade non-Hodgkin's lymphoma. Blood 89:3974-3979 1997.
62. Björkholm M, Ãsby E, Hagberg H, et al: Randomized trial of r-metHu granulocyte colony-stimulating factor (G-CSF) as adjunct to CHOP or CNOP treatment of elderly patients with aggressive non-Hodgkin's lymphoma (abstract 2655). Blood 94:599a, 1999.
63. Gómez H, Hidalgo M, Casanova L, et al: Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin's lymphoma: Results of a multivariate analysis. J Clin Oncol 16:2065-2069, 1998.
64. Doorduijn JK, van der Holt B, van Imhoff GW, et al: CHOP compared with CHOP plus granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin's lymphoma. J Clin Oncol 21:3041-3050, 2003.
65. Ãsby E, Hagberg H, Kvaløy S, et al: CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: Results of a Nordic Lymphoma Group randomized trial. Blood 101:3840-3848, 2003.
66. Intragumtornchai T, Sutheesophon J, Sutcharitchan P, et al: A predictive model for life-threatening neutropenia and febrile neutropenia after the first course of CHOP chemotherapy in patients with aggressive non-Hodgkin's lymphoma. Leuk Lymphoma 37:351-360, 2000.
67. Morrison VA, Weller EA, Habermann TM, et al: Patterns of growth factor (GF) usage and febrile neutropenia (FN) among older patients (pts) with diffuse large B-cell lymphoma treated (DLBCL) treated with CHOP or R-CHOP: An intergroup experience (CALGB 9793; ECOG-SWOG 4494) (abstract 3309). Blood 104:904a, 2004.
68. Morrison VA, Picozzi V, Scott S, et al: The impact of age on delivered dose intensity and hospitalizations for febrile neutropenia in patients with intermediate-grade non-Hodgkin's lymphoma receiving initial CHOP chemotherapy: A risk factor analysis. Clin Lymph 2:47-56, 2001.
69. Lyman GH, Morrison VA, Dale DC, et al: Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin's lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 44:2069-2076, 2003.
70. Clark OAC, Lyman GH, Castro AA, et al: Colony-stimulating factors for chemotherapy-induced febrile neutropenia: A meta-analysis of randomized controlled trials. J Clin Oncol 23:4198-4214, 2005.
71. Crawford J, Dale DC, Lyman GH: Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer 100:228-237, 2004.
72. Caggiano V, Weiss RV, Rickert TS, et al: Incidence, cost, and mortality of febrile neutropenia hospitalization (FNH) associated with chemotherapy. Cancer 103:1916-1924, 2005.
73. Chrischilles EA, Klepser DG, Brooks JM, et al: Effect of clinical characteristics on neutropenia-related inpatient costs among newly diagnosed non-Hodgkin's lymphoma cases during first-course chemotherapy. Pharmacotherapy 25:668-675, 2005.
74. Lyman GH, Kuderer NM: The economics of the colony-stimulating factors in the prevention and treatment of febrile neutropenia. Crit Rev Oncol Hematol 50:129-146, 2004.
75. Cosler LE, Sivasubramaniam V, Agboola O, et al: Effect of outpatient treatment of febrile neutropenia on the risk threshold for the use of CSF in patients with cancer treated with chemotherapy. Value in Health 8:47-52, 2005.
76. Lyman GH: Balancing the benefits and costs of colony-stimulating factors: A current perspective. Semin Oncol 30(suppl 13):10-17, 2003.
77. Zagonel V, Babare R, Merola MC, et al: Cost-benefit of granulocyte colony-stimulating factor administration in older patients with non-Hodgkin's lymphoma treated with combination chemotherapy. Ann Oncol 5(suppl 2):S127-S132, 1994.