Novel Antiangiogenesis Agent Shows Activity in Pretreated Glioblastoma
HOUSTON-Dynamic magnetic resonance imaging (MRI) has shown PTK787/ZK 222584 (PTK/ZK), an antiangiogenesis agent, to be capable of blocking vascular endothelial growth factor receptor (VEGFR)/VEGF activity in recurrent glioblastoma multiforme (ASCO abstract 315).
HOUSTONDynamic magnetic resonance imaging (MRI) has shown PTK787/ZK 222584 (PTK/ZK), an antiangiogenesis agent, to be capable of blocking vascular endothelial growth factor receptor (VEGFR)/VEGF activity in recurrent glioblastoma multiforme (ASCO abstract 315).
W.K. Alfred Yung, MD, Florence M. Thomas Professor of Cancer at M.D. Anderson Cancer Center in Houston, reported that vascular permeability was seen to decrease immediately after most patients received their first dose of PTK/ZK in a single-agent dose-escalation study. Although a rebound was observed, the vascular permeability index was not as high as at baseline for patients with stable disease or a partial response.
"We are seeing people stabilizing, and the tumor volume decreasing. This is very encouraging in a phase I study," Dr. Yung told ONI.
Failed Other Treatments
Among 29 patients treated so far, 2 have had their disease stabilize and 2 have had their tumors shrink. Although the patients had a life expectancy of 8 weeks when they entered the trial, Dr. Yung said that several have survived beyond 6 months.
All of the patients in the study had failed other treatments, and 83% had had adjuvant therapy. The mean time from diagnosis to patients entering the study was 13 months. Average age was 54.
The investigators chose to study PTK/ZK in glioblastoma multiforme because the malignancy is characterized by vascular proliferation and over expression of VEGF, Dr. Yung explained. He said that a parallel study is underway in colon cancer and sarcomas.
Progressive Dose Cohorts
Participants in the glioblastoma trial received the new agent in progressive three-to-six-patient cohorts, as researchers increased the dose from 500 mg per day to 2,000 mg per day (see Table 1). Dr. Yung said the optimal dose for a phase II trial is still being evaluated.
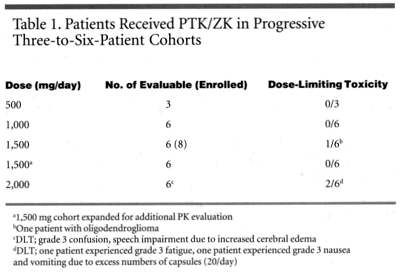
The phase I study has found no hematologic toxicity from PTK/ZK. Most of the toxicity seen at high doses has been fatigue, headache, and ataxia, according to Dr. Yung, who calls the regimen well tolerated.
The investigators also concluded that dynamic MRI is capable of demonstrating physiological or biological effect of blocking VEGFR/VEGF activity.